
Ozone has an oxidation potential of 2.07 V, almost higher than most other oxidative agents, making it highly reactive with all compounds. If you are not aware of ozone capability in water treatment or if you have a water pollution problem, read this paper to get more information about different applications of ozone water treatment.
Ozone Water Treatment (OWT) is an innovation in environmental science used for disinfection, organic and inorganic matter removal, and micropollutant oxidation. The initial reaction for the creation of ozone is an electrical and ionic excitation of molecular oxygen by a physical field, including electric discharges, ultraviolet radiation, or electrolysis. Ozone is a chemical reaction reagent with electrophilic and nucleophilic properties, which closely correspond to its resonance structure. Ozone can be potentially used as a broad-spectrum disinfectant and an enormous oxidant for water, owing to its excellent oxidation capacity with a wide range of pollutants. Various microorganisms can be effectively inactivated by ozone through cell lysis and destruction of nucleic acids.

As there are immense applications of ozone in water treatment, we are going to dig into its various uses in water and its efficiency and pros and cons of the use of ozone in water. So, follow this article to gain all you need to know about ozonation in the water industry.
1. Applications of Ozone Water Treatment
The main applications of ozone water treatment to treat surface and groundwater for drinking, Industrial, and agricultural purposes are listed below:
1.1- Disinfection
OWT was first used in 1886 by De Meritens for the disinfection of water. Ozone penetrates the cell membrane of bacteria and reacts with cytoplasmic substances. Furthermore, the efficiency of ozonation in the removal of poliomyelitis viruses, cysts, and other pathogens is confirmed. An ozone dose of 0.4 mg/l in 4 minutes is sufficient for disinfection by the ozonation of water.

1.2- Removal of Inorganic Compounds
Most of the time, ozone water treatment in inorganic removal is completely efficient. Ozone makes all inorganics into insoluble matters, which should be removed by the next treatment (e.g., filtration or coagulation). For example, ammoniacal nitrogen is oxidized into nitrate by ozonation and needs biological nitrification. Or metallic ions form their insoluble species and need filtration.
Note: In water containing bromide, try not to use ozonation because the behavior of bromide in water upon ozonation is preoccupant as it leads to the formation of hypobromous acid/hypobromite, a potential carcinogen (Zhang et al., 2022).
1.3- Application of Ozone in Organic Matter Removal
Significant organic matter is present in surface or groundwater, which can hurt water quality, i.e., color and odor. Also, the existence of organic matter causes bacterial regrowth, which leads to possible sanitary problems. Therefore, during treatment, it is advisable to remove as much natural organic matter as possible. Organic removal by ozone water treatment is reachable.
1.3.1. Removal of Color
Humic substances that lead to the color of the water will be removed by ozone water treatment. This may be due to a degradation of humic materials, which causes a loss of aromaticity. Phenolic and acidic compounds were detected as byproducts of ozonation. In practice, 50 mg/l color removal was complete, and at 60 mg O3/l, the final aldehyde concentration ranged between 0.72 and 1.02 mg/l (Mezzanotte et al. 2013).

1.3.2. Total Organic Carbon Removal by Ozonation
Total Organic Carbon (TOC) removal earns humic degradation. Ozonation of humic substances leads to the formation of small molecules, mainly aldehydes (formaldehyde, acetaldehyde, glyoxal, methylglyoxal) and carboxylic acids (formic, acetic, oxalic, glyoxylic, pyruvic, and keto malonic acids), which accumulate in the solution. It must be considered that results are of health concern since formaldehyde has shown evidence of mutagenicity and carcinogenicity. So, further water treatment is obligatory. In the study of Benincá et al. 2013, a substantial reduction of TOC (around 45%) was noticed in the water solution.
1.3.3. Increase in Biodegradability
Ozonation of organic matter produces low molecular weight compounds, which can be treated more easily in further steps. So, it can be concluded that before some main processes such as carbon filtration, ultrafiltration, or aeration.
1.3.4. Reduction in Trihalomethane Formation Potential
The presence of fulvic and humic acids in raw waters are the main predominant haloform, and their oxidation by chlorine leads to the production of TriHaloMethanes (THMs). Ozonation shows strong potential to degrade THMs into low molecular weight compounds that are less reactive towards chlorine.
1.4- Oxidation of Micro Pollutants
Micropollutants are another type of water contaminant that can be found in surface water. These micro-pollutants include chlorobenzenes, PolyChlorinated Biphenyls (PCBs), Polynuclear Aromatic Hydrocarbons (PAHs), and pesticides. These pollutants are harmful to human health and ecosystems (Camel and Bermond 1998). Efficient technology for removing these pollutants is required due to their recalcitrance toward biodegradation and toxicity in the environment. Several methods to remove these pollutants from water have been widely used, including catalytic ozonation, including O3/UV, O3/H2O2, and O3 combined with biological treatment and some other Advanced Oxidation Processes (AOPs) (Liu et al., 2018).
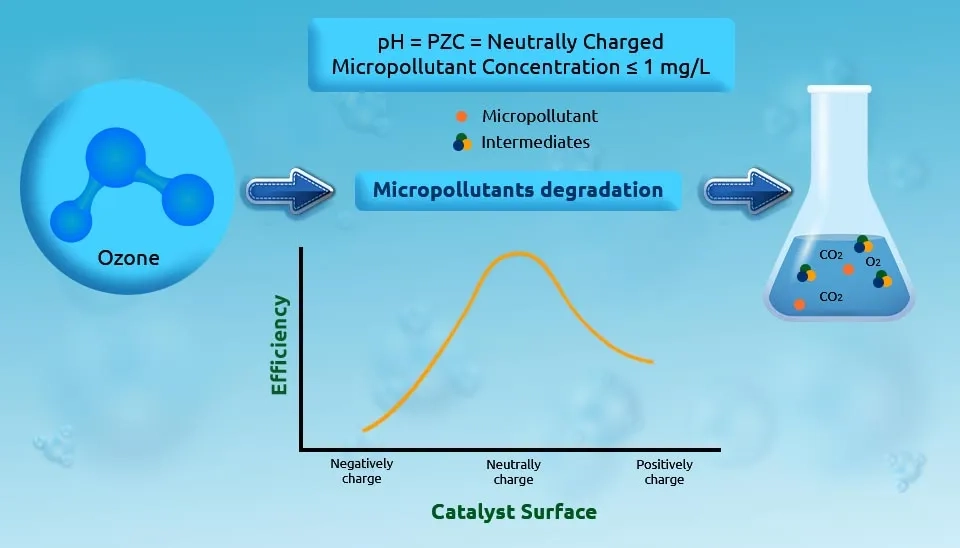
1.5- Pharmaceutical Removal
In recent years, Emerging Contaminants (EC), including pharmaceutical and personal care products, have attracted wide attention. After they are used in a variety of routes, the contamination and its intermediates shall be discharged into the environment. As a consequence, those antibiotics were frequently found in different aquatic environments. The conventional treatment processes are not very effective for the removal of antibiotics existing in pharmaceutical wastewater, while ozonation is used to degrade these contaminants.
Many medicinal products have aromatic properties that are potentially hazardous to animal or human health due to their persistence, bioaccumulation, nonbiodegradability, and toxicity. In the water treatment process, ozonation is an effective method for the degradation of pharmaceuticals (Paucar et al., 2019).
2. Pros and Cons of Ozone Water Treatment
Like all technologies, ozonation has its advantages and disadvantages, which will be discussed:
2.1. Pros
Speed: Disinfection of water by ozonation needs just a few minutes, while chlorination needs hours to kill pathogens.
Effectiveness: Ozone can remove a large era of contaminants, including organic, inorganic, pathogens, etc.
Reliable: Ozone has been used since 1906 and works properly on all water treatment plants.
Chemical free: There is no need to add chemicals during zonation, so there would be no change in the color or odor of water (Wei et al., 2016).
2.2. Cons
High costs: Equipment needs for ozone generation and operational costs are more than chlorination or other chemicals.
Toxicity: Different ozone reaction with bromate and halomethanes produces carcinogenic compounds
Low stability: ozone has a half-life of less than an hour in water, so chlorination should be used after ozonation for disinfection purposes (Dridger et al., 2001).
3. Conclusion
Ozone water treatment is one of the best choices for engineers dealing with polluted water, especially well water and surface water with high minerals and micropollutants. In this article, we investigate different applications of ozonation in the water industry. Results show that, firstly, to remove inorganic matter with ozonation, extra treatment such as a carbon filter is needed. Secondly, organic compounds can be degraded by ozonation in water solution, but it is better to apply ozonation with other methods, such as catalytic ozonation or other methods, namely, biological or physical treatment. Finally, for disinfection purposes, ozone can eliminate all pathogens in less than a minute. However, to stop the regrowth of microbes and other pathogens, chlorine must be introduced to pipelines. Moreover, the pros and cons of ozonation systems are discussed for better decisions by engineers.

