
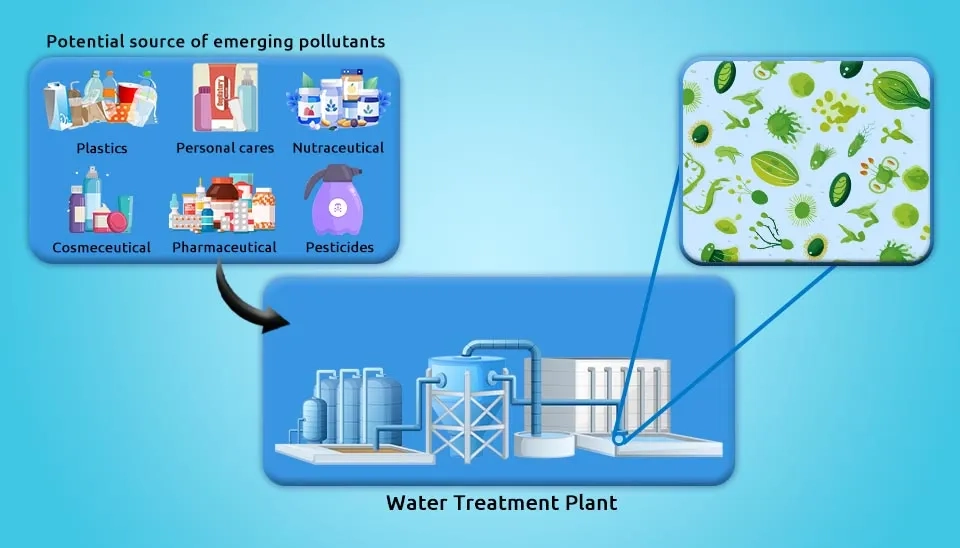
Emerging pollutants (ECs), including 1) nutrients, 2) heavy metals, 3) antibiotics or pharmaceuticals, 4) Polycyclic Aromatic Hydrocarbons (PAHs), 5) pesticides, 6) herbicides, 7) manure, 8) insecticides, and 9) surfactants, have become a developing concern in recent years due to their potential adverse effects on human health and the environment (Gondi et al., 2022; Ghosal et al., 2016; Wood et al., 2016; Kumar et al., 2015; Chang et al., 2015).
Find out how bacteria can be combined with microalgae for a synergistic treatment approach: Microalgae-Bacteria Consortia for Wastewater Treatment.
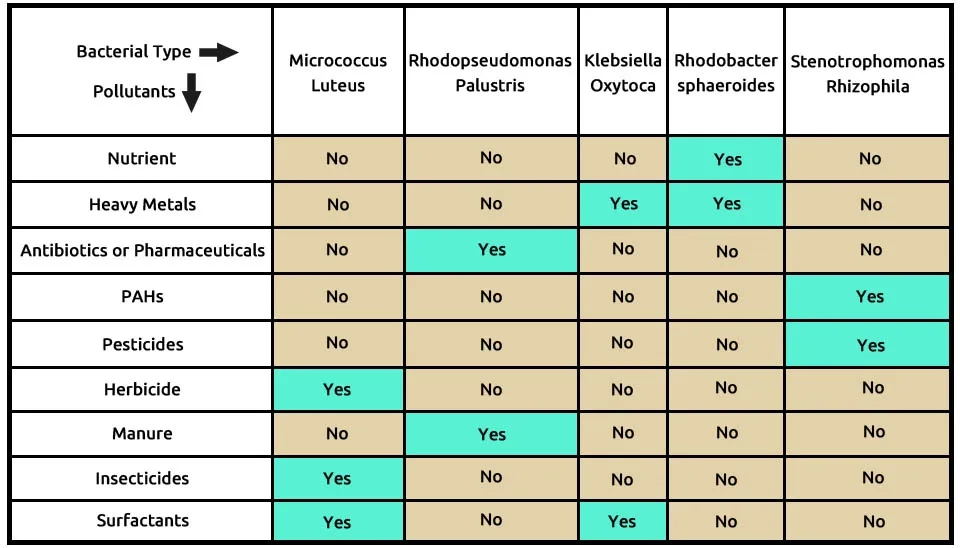
Table. 1. Details the pollutant removal capabilities of 82 bacterial strains, based on 145 studies. The table indicates which bacteria effectively address nine categories of emerging contaminants, including nutrients, heavy metals, antibiotics, and pesticides.(Brief Table).
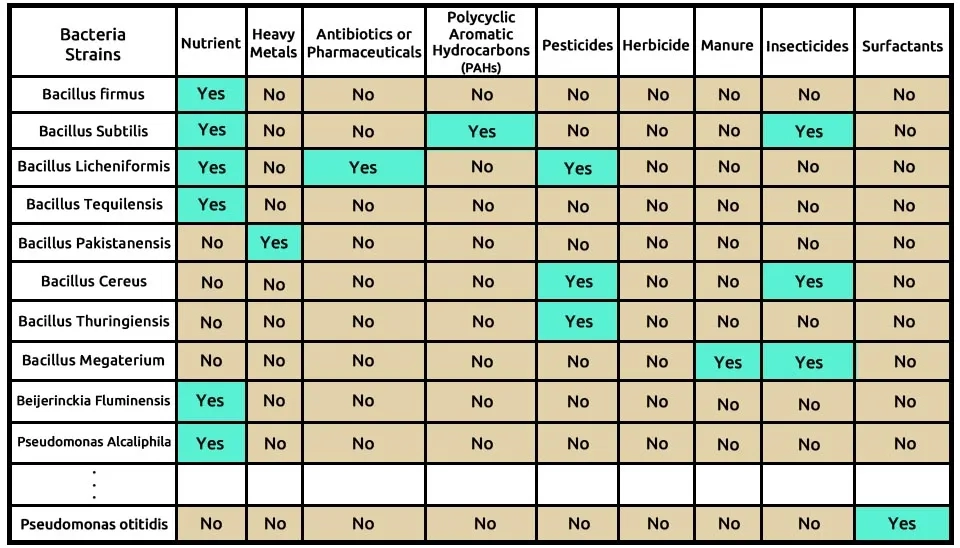
Download Full Detailed Table of Table. 1. Pollutant Removal Capabilities of Selected Bacterial Strains.
Are you prepared for a revolutionary solution to emergency contamination? Imagine a world where a powerful bacterial consortium swoops in to swiftly and effectively eliminate harmful substances, ensuring a safe and clean environment for all. Prepare to be amazed by the incredible potential of this cutting-edge technology in combating emergencies with precision and speed. Follow our article to the end for a comprehensive understanding of the subject, acquaint yourself with these compounds, or even create your own formulations tailored to your local conditions. These pollutants are produced globally due to agricultural use of insecticides and are figured to encounter a slight increment from around 704.48 thousand metric tons in 2023 to around 712.16 thousand metric tons in 2027. Surfactants have been increasing from 15.93 million tons used in 2014 to an expected 24.19 million tons used in 2022 (Palmer and Hatley, 2018). The growing global consumption of medicines also necessitates effective Pharmaceuticals Wastewater Treatment.
Every year, more than 4 million tons of pesticides are produced around the world (Mosalaei Rad et al., 2022). These pollutants are often in wastewater and can be difficult to remove using traditional treatment methods, such as Primary Wastewater Treatment. Bacterial consortia have emerged as a promising approach for the removal of these emerging pollutants from wastewater with MBBR wastewater treatment.
Bacterial consortia can target multiple pollutants at once and degrade complex pollutants into simpler compounds that are easily removed from wastewater by combining different species of bacteria. This article will explore the use of bacterial consortia for the removal of nine pollutants. These bacterial consortia are suggested by the author, and you can use the link below to get your desired combination.
1. PKPR Bacterial Consortium
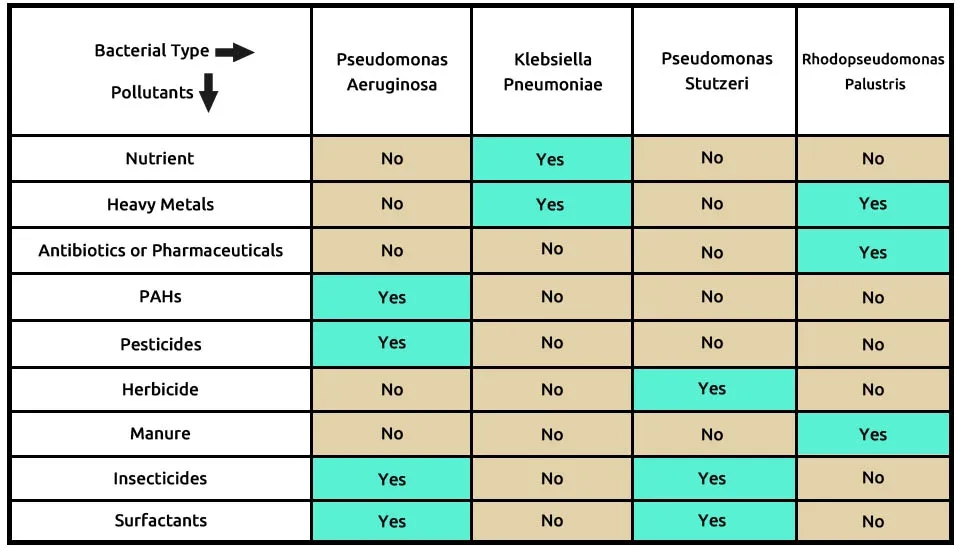
Table 2 shows the first combination with four bacterial consortia, including P (Pseudomonas aeruginosa), K (Klebsiella pneumoniae), P (Pseudomonas stutzeri), and R (Rhodopseudomonas Palustris). Pseudomonas aeruginosa can remove many contaminants, such as PAHs, pesticides, insecticides, and surfactants. Klebsiella pneumoniae can remove contaminants such as nutrients and heavy metals from Municipal Wastewater. Pseudomonas stutzeri breaks down other pollutants, including herbicides and insecticides, and Rhodopseudomonas palustris biodegrades heavy metals, antibiotics or pharmaceuticals, and manure. The composition of bacteria, including Pseudomonas aeruginosa, Klebsiella pneumoniae, Pseudomonas stutzeri, and Rhodopseudomonas palustris, in the removal of 9 pollutants from ECs is presented in Table 2.
Jabeen et al. (2015) degraded Profenofos (PFF), an Organophosphate (OP) insecticide, using a bacterial consortium consisting of Achromobacter xylosoxidans, Pseudomonas aeruginosa, Bacillus sp., and Citrobacter koseri. The best conditions for breaking down PFF were found to be a pH of 6.83, a temperature of about 35°C, and an inoculum size of 0.59 g L-1. This led to the highest level of PFF degradation at 93.39%. In two glass flasks, they put 100 ml sterile Minimal Salt Medium (MSM) and added 50 and 100 mg L-1 PFF. Then, they were incubated in a rotary shaker at 37°C and 100 rpm. Two weeks after incubation, 5 ml of suspension from each sample was transferred to flasks with new MSM, and 50 and 100 mg L−1 pesticides were added. Li et al. (2020) enhanced the degradation of two pyrene PAHs degrading strains, Pseudomonas aeruginosa and Achromobacter sp. The optimized dosage and the perfect initial pHs were 1.4 g L−1 and 5.5, respectively. Results show that Pseudomonas aeruginosa had a better effect than Achromobacter sp. in bacterial growth. When both 600 mg/L pyrene and 1.4 g/L sodium citrate were used together, 74.6% of the pyrene was broken down. This is 1.57 times, 2.06 times, and 3.89 times more than when mix-culture strains, single Pseudomonas aeruginosa, and single Achromobacter were used without sodium citrate. The inoculums were prepared by mixing Pseudomonas aeruginosa or Achromobacter cultures with MSM medium in a 250 mL orbital shaker set at 30°C and 200 rpm for 14 days. Then, the fermented liquid was centrifuged at 8000 rpm at 4°C for 20 min, and the cells were collected, washed, and resuspended in 0.9% saline solution to prepare the seed culture.
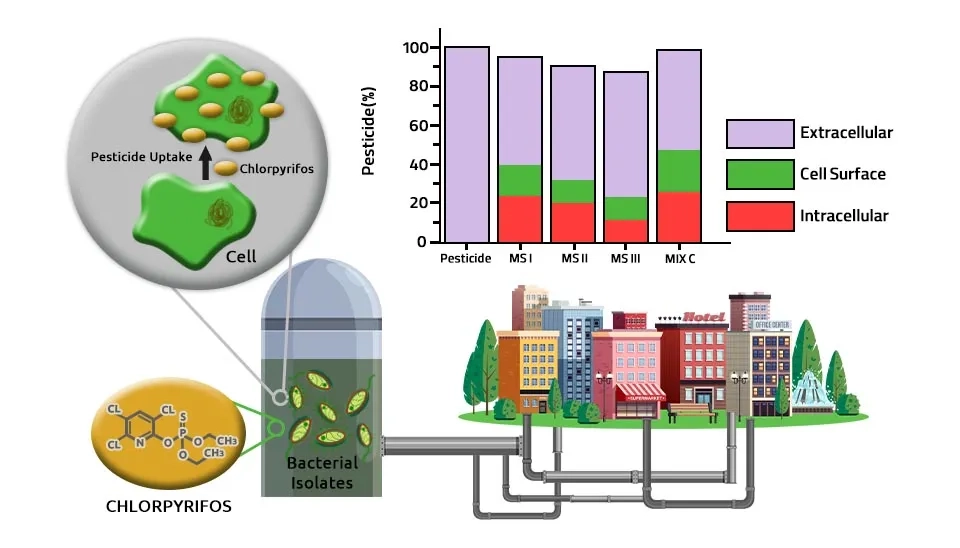
Chaturvedi and Kumar (2011) investigated isolating and identifying Sodium Dodecyl Sulfate (SDS) surfactants degrading bacteria from different detergent-contaminated ponds. In identical conditions, the rate of SDS degradation varied from 97.2% to 19.6% after 12 h incubation. Bacteria isolates belonged to Pseudomonas aeruginosa, Pseudomonas mendocina, Pseudomonas stutzeri, Pseudomonas alcaligenes, Pseudomonas pseudoalcaligenes, Pseudomonas putida and Pseudomonas otitidis respectively. They put 1 ml of pond water in 100 ml of sterilized Phosphate Buffered Medium (PBM) supplemented with SDS (1 g/l) in a culture flask and incubated at 30°C and 120 rpm in a shaker. After the bacteria grew for 3–4 days, 1 ml of the culture was transferred to fresh PBM with added SDS (1 g/l). Song et al. (2015) identified a new strain of bacteria, Pseudomonas aeruginosa, that can degrade the insecticide fenpropathrin. Up to 92.3 % of fenpropathrin (50 mg l-1) was removed by P. aeruginosa strain at 30°C and pH 7 within 7 days. They inoculated 1 g of soil on the Luria-Bertani (LB) medium and incubated at 30°C for 2 days. The well-isolated colonies were moved into an MSM with 50 mg l-1 fenpropathrin added at 30°C in a 250 ml conical flask for 7 days. The transfer was conducted five times successively until the fenpropathrin concentration gradually increased to 800 mg l-1. Shabbier et al. (2018) found three bacteria from domestic sewage, Pseudomonas aeruginosa, Enterobacter ludwigii, and Enterobacter cloacae, can grow in the presence of the organo-phosphorous pesticide (chlorpyrifos). Up to 50 % of chlorpyrifos was degraded by bacteria strains at 37°C and pH 7.4 after 30 days. 10 % sewage sludge was added to LB broth supplemented with 0–50 mg/ml of chlorpyrifos and incubated at 37°C overnight. Samples from each treatment were added to the LB medium for 7 days.
Gaur et al. (2018) utilized different ligninolytic enzymes from novel bacterial strains of Klebsiella pneumoniae for the aim of better degradation and decolorization of wastewater. In lignin degradation and decolorization, various consortia of Klebsiella pneumoniae were obtained 74–83% at 5–8 days. The effluent sample was collected from the Pulp and paper industry (which often require Industrial Wastewater Treatment) contained a significant amount of chlorophenols and lignin. Lignin Basic Media (LBM) was used for the isolation of bacteria with 1.5% w/v agar and was added glucose into the media after autoclaving 0.1% (w/v). Aransiola et al. (2017) investigated the potential of Klebsiella species, including Klebsiella edwardsii, Klebsiella oxytoca, and Klebsiella pneumoniae. They isolated from diesel-polluted soil by bioremediating the heavy metals (Cr, Cd, Cu, and Ni) present in effluent water at 16 days. The water bio-remediated for heavy metals is obtained 81–99%. They used 20 ml molten sterile Eosin Methylene Blue (EMB) agar to culture the bacteria. The culture plate was carefully turned to mix the cell suspension with the medium. They were incubated at 35°C for 48 h.
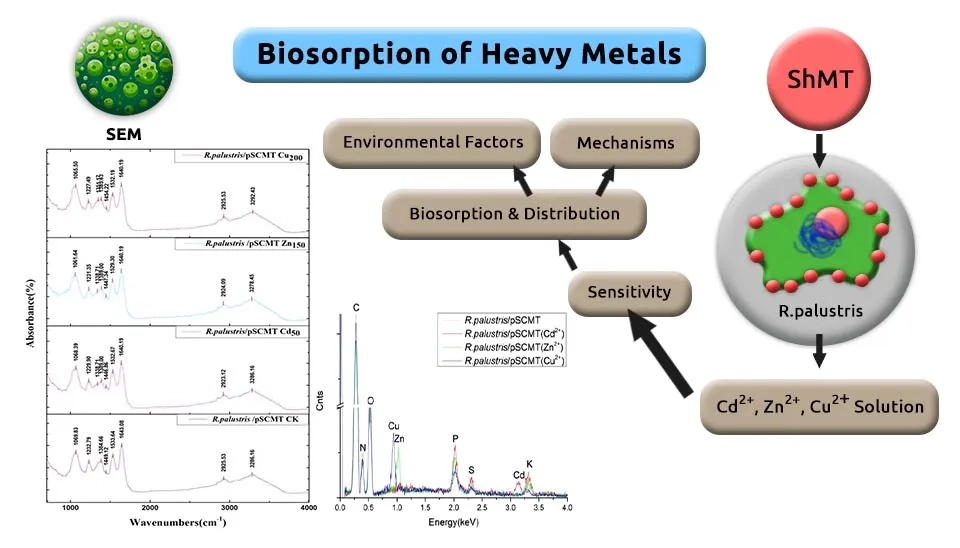
Saikia et al., (2005) completely degraded β-cyfluthrin pesticide using Pseudomonas stutzeri at 20 days. They used a Mineral salt medium for the isolation of bacterial strain at a pH of 7.0. After mixing well, the preparations were incubated in Petri dishes at 28°C for 30 days. Paulo et al., (2013) identified two denitrifying bacteria, including Pseudomonas stutzeri and Pseudomonas nitroreducens, can consume SDS surfactants as the substrate from an activated sludge reactor of a Wastewater Treatment Plant (WWTP) with Anaerobic-Anoxice-Oxic (A2/O) steps. Removal efficiency SDS obtained 99% at 16h. The samples were prepared in 120 mL serum bottles and contained 50 mL of medium and a gas phase of helium (1.4 atm). Batch enrichments were incubated at 30°C and a pH of 7.3 ± 0.1. Jia et al., (2022) used Rhodopseudomonas palustris bacteria, along with Metallothionein (MT), to investigate its removal and biosorption capacity for Cd, Zn, and Cu in the medium and can be compared to physical methods like Reverse Osmosis. Rapid removal was 92.1% of 25 mg/L Cd within 30 mins. The maximum biosorption capacities calculated for Cd, Cu, and Zn by the Langmuir model were 224.22, 233.64, and 99.8 mg/g, respectively.
Riquelme et al., (2018) investigated Pseudomonas aeruginosa and Klebsiella pneumoniae adaptation and were shown to have the same ecological. Pseudomonas aeruginosa and Pseudomonas stutzeri are from the Pseudomonas family and completely are compatible. Wittayapipath et al., (2019) used Pseudomonas stutzeri and Klebsiella pneumoniae to study the mechanism of action that could be related to changes in this bacterial biochemical composition. Xanthyletin and the secondary metabolites from microalgae exerted anti-P. insidiosum activity that completely changed the proteins in P. insidiosum. Strains were incubated on a rotary shaker at 200 rpm at 37°C for 3 days. According to research, bacterial consortium for emerging contaminations removal in this composition can grow together in the environment. However, the outcome of their interactions can vary depending on factors such as nutrient availability, competition for resources, and production of antimicrobial compounds. In some cases, specific bacterial strains may outcompete others, while in other cases, they may coexist peacefully. It ultimately depends on the particular conditions of the environment in which they are growing.
2. PCBEP Bacterial Consortium
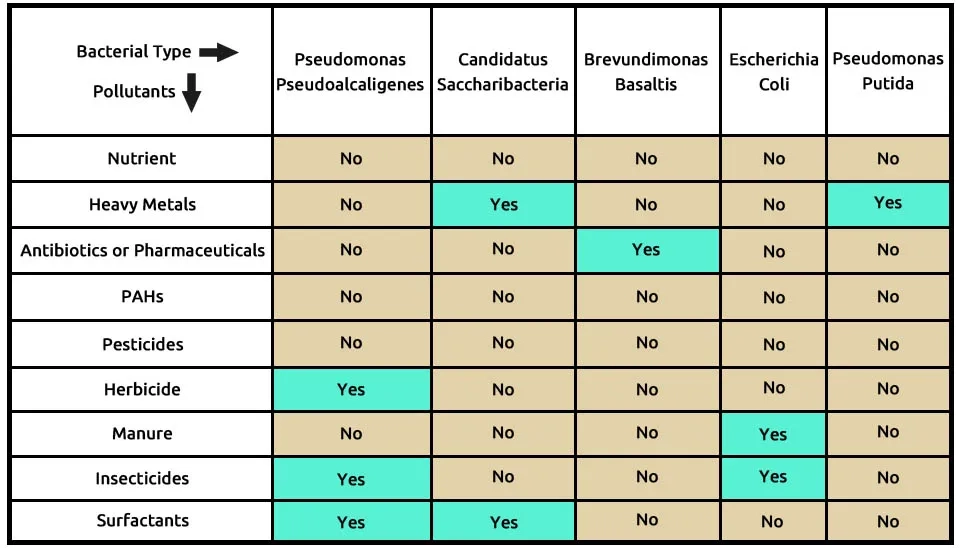
The second suggested combination is the five bacterials, including P (Pseudomonas pseudoalcaligenes), C (Candidatus saccharibacteria), B (Brevundimonas basaltis), E (Escherichia coli), and P (Pseudomonas putida), that completely remove these 9 contaminants, according to Table 3. Pseudomonas pseudoalcaligenes is able to remove many pollutions, such as herbicides, insecticides, and surfactants. Heavy metals and antibiotics or pharmaceuticals are removed by Pseudomonas putida and Brevundimonas basaltis, respectively. According to Table 3, other pollutions were removed using Candidatus Saccharibacteria and Escherichia coli. The composition of bacteria, including Pseudomonas pseudoalcaligenes, Candidatus Saccharibacteria, Brevundimonas basaltis, Escherichia coli, and Pseudomonas putida in the removal of 9 pollutants of ECs, is presented in Table 3.
Xiujie et al., (2019) combined Partial denitrification (NO3--N → NO2--N) with Anaerobic Ammonium Oxidation (ANAMMOX) to achieve nitrogen removal with a low C/N ratio and low energy consumption using Candidatus saccharibacteria a 120 days run. They investigated three different acclimation conditions, namely, R1 (Sequencing Batch Reactor (SBR) under anoxic conditions), R2 (SBR under alternating anoxic/aerobic conditions), and R3 (SBR under low-intensity aeration). In pH 9.5, the nutrient is completely removed in 10 hours. The working volume was 4.0 L at a constant temperature of about 25°C with an initial pH of 7.3, C/N = 3, and a rotational speed of 90 rpm. Herzog et al., (2013) isolated nine different bacteria species capable of activated sludge communities, including Brevundimonas basaltis, for Sulfamethoxazole (SMX, sulfonamide antibiotic) completely removed after 10 days. Bacterial cultures were successfully cultivated and isolated from activated sludge communities. 200 μL was plated on solid R2A-UV to inhibit the growth of non-resistant bacteria and foster the growth of potential SMX-resistant/biodegrading organisms.
Zhao et al. (2019) used an N2-fixing bacterium, Escherichia coli, for thiacloprid neonicotinoid insecticide removal (97%) after 10 min. Escherichia coli was incubated in a rotary shaker at 220 rpm with a temperature of 37°C. Safonova et al. (2004) conducted the ability of bacterial consortium, including Bacillus coagulans, Bacillus licheniformis, Bacillus pumilus, Bacillus subtilis, Nitrosomonas sp., and Pseudomonas putida, in bioremediation of wastewater. They found that these bacterial consortiums can reduce BOD 71.93%, 64.30% Chemical Oxidation Demand (COD), TSS 94.85%, and 88.58% of ammonia in 10 days. They applied an experimental method for the bioremediation of wastewater with a Completely Randomized Design (CRD). Sampling time and bacterial consortiums consisted of 8 levels.
Kim et al. (2003) examined Escherichia coli strains expressing either toluene dioxygenase from Pseudomonas putida or biphenyl dioxygenase from Pseudomonas pseudoalcaligenes for their ability to catalyze flavones. Pseudomonas pseudoalcaligenes and Pseudomonas putida are from Pseudomonas family and completely are compatible. Bacteria were grown in 10 ml Luria Bertani (LB) broth medium with 50 mg ampicillin /ml for the seed culture at 37°C with 250 rpm and phosphate buffer (pH 7.5) for 6 h. Chen et al. (2023) presented comparable nitrogen removal ability using bacterial consortium, including Rhodobacter, Saccharomycetales Gymnopilus dilepis, Lactobacillus, and Pseudomonas. During the domestication process, the Spent Mushroom Substrates (SMS) fermentation broth-feeding treatment presented comparable nitrogen removal ability (74.44%) with a commercial carbon source group (77.99%). The temperature was held at 30°C with 95% synthetic wastewater and 5% SMS fermentation liquid initially at a 40 rpm rotation speed, and each react cycle time was about 8 h. Molina-Santiago et al. (2017) investigated interspecies cross-talk between co-cultured Pseudomonas putida and Escherichia coli. Co-culture of these microbes significantly alters transcriptional profiles. E. coli, in response to the presence of P. putida, can produce acetate as a mechanism. Strains cultivated at 30°C in LB medium had a turbidity of 0.6 at 600 nm. According to studies, Pseudomonas pseudoalcaligenes, Candidatus Saccharibacteria, Brevundimonas basaltis, Escherichia coli, and Pseudomonas putida can grow together under certain conditions. Competition for nutrients and space may occur between the different species, affecting their growth rates. Proper management and monitoring of the culture system would be required to achieve successful co-cultivation of these bacterial species.
3. ABBBE Bacterial Consortium
The third suggested combination used the 5 bacterial strains, including A (Acinetobacter calcoaceticus), B (Bacillus licheniformis), B (Bacillus megaterium), B (Bacillus subtilis), and E (Exiguobacterium aurantiacum) that completely remove these 9 contaminants, according to Table 4. Bacillus licheniformis removes 4 pollutants such as nutrients, antibiotics or pharmaceuticals, pesticides, and surfactants from wastewater. Bacillus megaterium, Bacillus subtilis, and Exiguobacterium aurantiacum help to remove better insecticides together. Herbicides, PAHs, and manure are removed by the bacterial consortium, according to Table 4. The composition of bacteria, including Acinetobacter calcoaceticus, Bacillus licheniformis, Bacillus megaterium, Bacillus subtilis, and Exiguobacterium aurantiacum in the removal of 9 pollutants of ECs, is presented in Table 4 , especially with Membrane Bioreactor (MBR) technology.
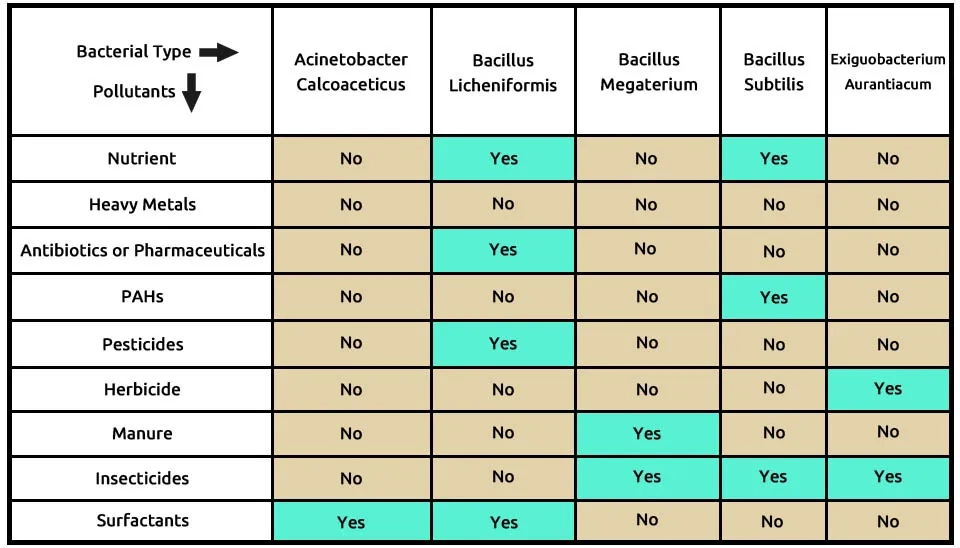
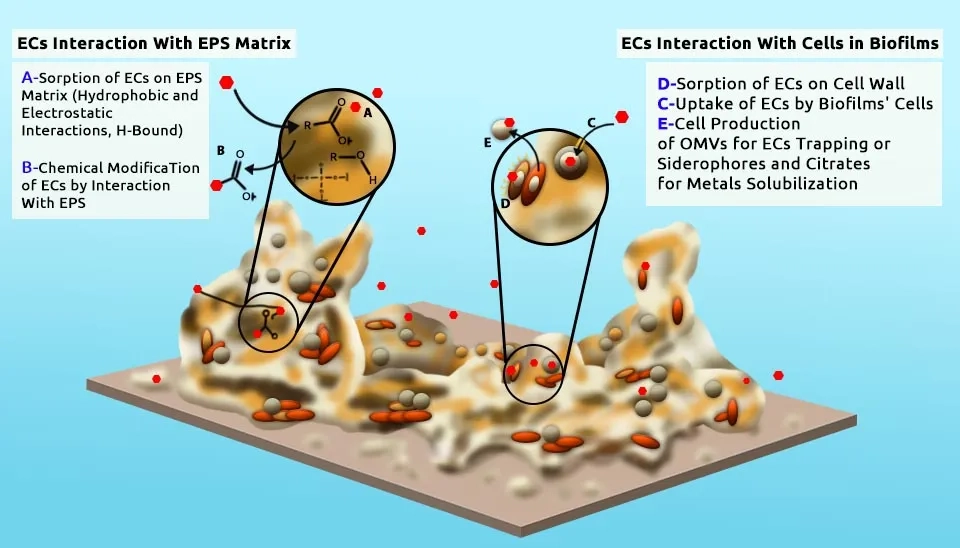
Abboud et al. (2007) degraded the anionic surfactants Linear Alkylbenzene Sulfonate (LAS) and SDS using a consortium of mixed facultative anaerobes, Acinetobacter calcoaceticus, and Pantoea agglomerans, an alternative to Dissolved Air Flotation (DAF) wastewater treatment, They found a complete degradation of 4000 ppm SDS biomass within 120 h of incubation time, while at 150 h, it was capable of degrading only 60% of 300 ppm LAS biomass under growth conditions in nutrient broth medium at a temperature of 30°C; pH 8.5; and agitation rates of 250 rpm. Akbar et al. (2015) identified three bacterial strains isolated from agricultural soil, including Acinetobacter calcoaceticus MCm5, Brevibacillus parabrevis FCm9, and Sphingomonas sp. RCm6 is highly efficient in degrading cypermethrin insecticide and other pyrethroids. These bacterial strains can degrade more than 85 % of cypermethrin at 10 days in cultivation conditions, including MSM, pH 6.8–7.0, 1.5 % of agar, and 30°C at 150 rpm. Jaiswal et al. (2019) investigated the toxicity of Organophosphate (OPs) insecticides Monocrotophos (MCP) and Chlorpyrifos (CLS) on Plant Growth Promoting (PGP) properties and seed germination of brinjal, tomato, and okra vegetables immunized by Microbacterium hydrocarbonoxydans, Stenotrophomonas rhizophila, Bacillus licheniformis and Bacillus cereus. Various bacterial strains showed 54–90% of insecticide degradation at a 50–150 mg/kg concentration within 5 days. Isolation of microbes was performed by serial dilution and plating method on a Petri Plate containing various media.
Sonune and Garode. (2018) isolated a total of 34 bacterial isolates, including Bacillus licheniformis, from municipal wastewater, which consists largely of greywater and sludge samples and showed a reduction in Biochemical Oxidation Demand (BOD), COD, nitrate, and phosphate by 50.65%, 20%, 56.25%, and 31.13% respectively after 72 h. Also, they showed that all the isolates were able to produce lipase and protease. Screened isolates were inoculated for protease production on milk nutrient medium, including (w/v) peptic digest of animal tissue 0.5%; beef extract 0.15%; yeast extract 0.15%; NaCl 0.5%; egg yolk 1%; agar 1.5%, pH 7.0 ± 0.2 and incubated at 37°C for 24–72 h. Akbar et al. (2015) identified three potential Cypermethrin (CM) insecticide degrades using Ochrobactrum anthropi, Bacillus megaterium, and Rhodococcus sp. JCm5 degraded 86–100% of CM (100mgL-1) within 10 days. The pH and temperature of the samples were 6.9 and 30°C at 150 rpm with 20.6% moisture content, 38.5% water holding capacity, and 4.3% organic matter content.
Xiao et al. (2014) reported a novel bacterial strain, Bacillus subtilis, which was isolated from activated sludge, showed high efficiency in degrading (89.4%) at 50 mg L−1 the pyrethroid insecticide beta-cypermethrin (beta-cp) within 7 days. The optimal conditions for insecticide degradation were obtained to be 34.5°C, pH 6.7, and inocula amount 0.11 g dry wt L−1 using response surface methodology. Safitri et al. (2015) conducted consortium of Bacillus pumilus, Bacillus subtilis, Bacillus coagulans, Nitrosomonas sp., and Pseudomonas putida with an inoculum concentration of 5% the most effective in reducing BOD 71.93%, 64.30% COD, TSS 94.85%, and 88.58% of ammonia in bioremediation of wastewater in 420 hours. Mandree et al. (2021) completely degraded Polycyclic Aromatic Hydrocarbons (PAHs), including naphthalene, phenanthrene, and pyrene, using Bacillus spp. such as Bacillus velezensis, Bacillus subtilis after 74 days. Culture collection was incubated at 32°C, pH 6.8, and 180 rpm using a shaker.
LO´ PEZ et al. (2005) studied the growth and capacities for various contaminations removal (90–100%) such as organochlorinated insecticides (aldrin and lindane), organophosphorus insecticides (dimethoate, methyl-parathion, and methylation), s-triazine herbicides (simazine and atrazine), fungicide (captan) and diflubenzuron (1-(-4-chlorophenyl)-3-(2,6-difluoro benzoyl urea) by bacterial strains, including Pseudomonas pseudoalcaligenes, Micrococcus gluteus, Bacillus sp., and Exiguobacterium aurantiacum after 28 days. The inoculated agar plates were incubated at 28°C with continuous agitation (150 rpm) and pH 7.2 before colonies were counted. Bacterial strains Bacillus licheniformis, Bacillus megaterium, and Bacillus subtilis are entirely compatible with the Bacillus family. The compatibility of the Acinetobacter calcoaceticus and Exiguobacterium aurantiacum bacteria with Bacillus spp. in optimum cultivation conditions should be investigated.
4. MRKRS Bacterial Combination
According to Table 5, we suggest the finished combination of bacterial strains, including M (Micrococcus luteus), R (Rhodopseudomonas Palustris), K (Klebsiella oxytoca), R (Rhodobacter sphaeroides),and S(Stenotrophomonas rhizophila), that can remove 9 pollutants. Klebsiella oxytoca,such as Rhodobacter sphaeroides, can remove heavy metals and nutrients from municipal wastewater and Surfactants. Micrococcus luteus is able to take herbicides, insecticides, and surfactants alone. Rhodopseudomonas Palustris removed antibiotics or pharmaceuticals and manure. Also, Stenotrophomonas rhizophila breaks down PAHs and pesticides only. The composition of bacteria, including Micrococcus luteus, Rhodopseudomonas Palustris, Klebsiella oxytoca, Rhodobacter sphaeroides,and Stenotrophomonas rhizophila in the removal of 9 pollutants of ECs, is presented in Table 5.
Eniola, 2012 used various consortia of pure cultures of Alcaligenes odorans, Citrobacter diversus, Micrococcus luteus, and Pseudomonas putida for the biodegradation (86%) of Linear Alkylbenzene Sulfonate (LAS) surfactant after 16 days. Bacterial strains cultivated in LAS Mineral Medium (LMM), pH 7, at room temperature (28°C ± 3) on an orbital shaker at 100 rpm. Khleifat. (2006) degraded Sodium Lauryl Ether Sulfonate (SLES) surfactants were isolated from a wastewater treatment plant using two bacterial consortia, Acinetobacter calcoacetiacus and Klebsiella oxytoca and Serratia odorifera and Acinetobacter calcoacetiacus with degradation ability in a range between 25–100% after 144 hr. The culture was incubated at 37°C and under a 150 rpm shaking rate at pH 7.5, and the Nutrient Broth (NB) and Agar Medium (AM) were used for the isolation of bacteria. HE et al. (2010) used Purple Nonsulfur Bacteria (PNSB) such as Rhodobacter sphaeroides wastewater treatment as clean technology and generated single-cell protein while degrading pollutants. The Rhodobacter sphaeroides could grow well under natural conditions, and its treatment of soybean wastewater obtained zero order reaction, and COD reduction was 96% after 10 d. The culture was a 500 mL glass beaker, which was placed in a reciprocating thermostatic shaker shaking at 200 rpm at a temperature range of 28–30 °C, and pH was around neutral 7.0 ± 0.1.
Conclusion
These suggested cutting-edge bacterial consortium for emerging contaminations removal are specifically engineered to target a wide range of contaminants, including 1) nutrients, 2) heavy metals, 3) antibiotics or pharmaceuticals, 4) Polycyclic Aromatic Hydrocarbons (PAHs), 5) pesticides, 6) herbicides, 7) manure, 8) insecticides, and 9) surfactants. Its suggested unique composition allows for the rapid breakdown and degradation of ECs, resulting in a swift and thorough remediation process. By harnessing the natural capabilities of different bacterial strains working in synergy, this technology can effectively neutralize even the most stubborn contaminants, restoring ecosystems and protecting human health. Furthermore, the use of this innovative bacterial consortium is not only highly effective but also environmentally friendly. Unlike traditional remediation methods such as Coagulants in Wastewater Treatment, that may involve harsh chemicals or disruptive processes, this approach relies on natural biological processes to achieve its goals. According to the research conducted, these proposed compositions of microalgae(for Microalgae Wastewater Treatment) have removed all pollutants from that family examined in the research, and more detailed tests are needed to remove all the pollutants. The utilization of a powerful bacterial consortium represents a groundbreaking advancement in the remediation of ECs. Embrace this innovative technology as a reliable and efficient solution for addressing emergency contaminations and safeguarding the health and well-being of communities around the world.