Having access to clean and safe water has been recognized as an essential human right by the United Nations (UN, 2010). An important step in providing clean water without the risk of waterborne disease is disinfection. In the disinfection process, pathogenic microorganisms are eliminated to ensure that consumers receive safe water through distribution networks. Chlorine-based disinfectants have been widely used because of their high efficiency, reasonable costs, and stability in distribution networks. However, the discovery of Disinfection ByProducts (DBPs) as a result of chlorine-based treatments raised a lot of concern, which led to the use of other disinfection technologies such as ozonation and ultraviolet that are more costly. Nevertheless, it was seen that the formation of disinfection byproducts still occurred even after using non-chlorinated disinfectants (Gilca et al., 2020).
In this article, we will review the most important DBPs that can be formed in our drinking water and the factors influencing their production. Then, we will discuss the adverse effects of disinfection byproducts on human health. In the next part of this article, we will address some critical steps to consider for reducing the production of DBPs in the water treatment process. Finally, we will mention the DBP regulations that organizations and agencies have adopted.
Table. Maximum contaminant levels of important disinfection byproducts based on various regulations and guidelines (μg/L) (Health Canada, 2022; Roque et al., 2023).
Disinfection Byproducts | Disinfectant | US Regulations |
Total Trihalomethanes (TTHMs) | Chlorine | 80 |
Haloacetic Acids (HAA5) | Chlorine | 60 |
Chlorite | Chlorine dioxide | 1000 |
Bromate | ozone | 10 |
Disinfection Byproducts | Disinfectant | EU Regulations |
Total Trihalomethanes (TTHMs) | Chlorine | 100 |
Haloacetic Acids (HAA5) | Chlorine | 60 |
Chlorite | Chlorine dioxide | 250 |
Chlorate | Chlorine dioxide | 250 |
Bromate | ozone | 10 |
Disinfection Byproducts | Disinfectant | Canadian Guideline |
Total Trihalomethanes (TTHMs) | Chlorine | 100 |
Haloacetic Acids (HAA5) | Chlorine | 80 |
Chlorite | Chlorine dioxide | 1000 |
Chlorate | Chlorine dioxide | 1000 |
Bromate | ozone | 10 |
1. What Are Disinfection Byproducts (DBPs)?
Disinfection byproducts is a term that refers to unintended products that are formed when disinfectants such as chlorine react with Natural Organic Matter (NOM) or inorganic precursors (e.g., bromide, iodine, and nitrate) (Hebert et al., 2010). About 50 years ago, in 1974, Johannes Rook discovered the formation of disinfection byproducts in treated water for the first time. Rook identified four TriHaloMethanes (THMs) in chlorinated water, including chloroform, bromoform, bromodichloromethane, and dibromochloromethane. He suggested that these 4 THMs (also known as THM4) are generated as a result of reactions between humic acid and chlorine. Since then, thousands of studies have been conducted, and more than 600 DBPs have been discovered (DeMarini, 2020; Rook, 1974).
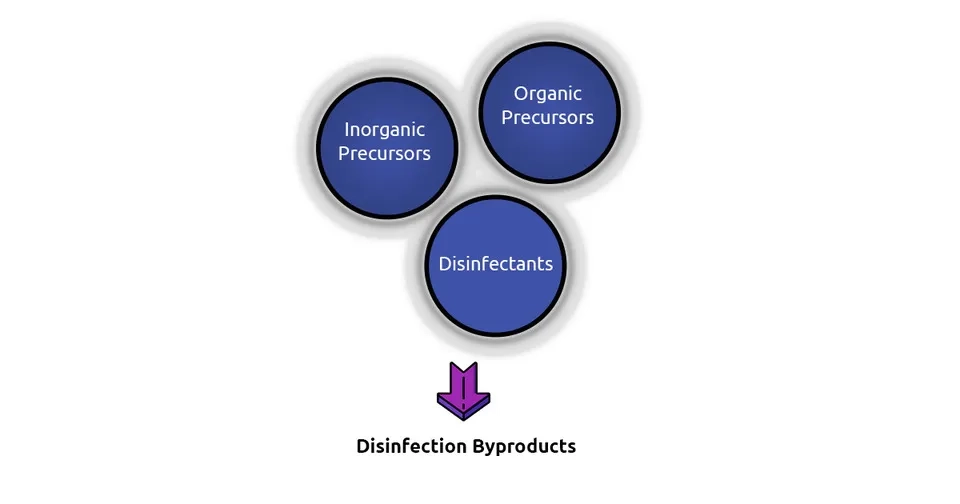
2. Classification of DBPs
While DBPs comprise a wide range of chemicals, we will introduce you to some of the most occurring groups in this section, including THMs, HAAs, inorganic DBPs, and other halogenated DBPs.
2.1. Trihalomethanes (THMs)
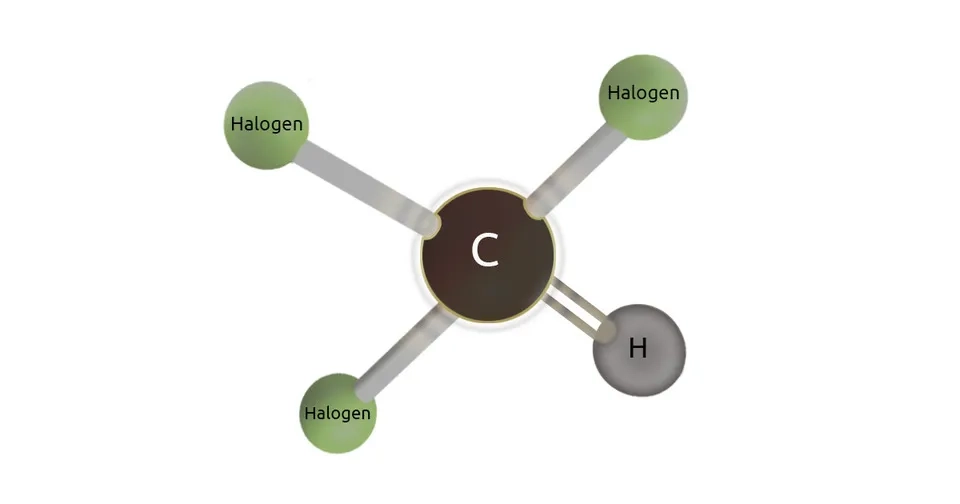
Trihalomethanes are the first group of DBPs that were discovered. THMs are a group of chemicals in which three of four hydrogen atoms are replaced with halogens (i.e., chlorine, bromine, and iodine). Trichloromethane, also known as chloroform, is the most common trihalomethane and accounts for 90% of THMs concentration. THMs form as a result of the presence of natural organic matter in humic form. These organic substances can be found in natural water, and they are considered to be harmless. However, their reaction with chlorine can form harmful compounds that are listed as primary pollutants by the Environmental Protection Agency (EPA). THMs are the first common byproducts in treated water and there is a great concern regarding their potential carcinogenic effects even at very low concentrations(Carvalho et al., 2007; Durmishi et al., 2015)
2.2. Haloacetic Acid (HAAs)
HaloAcetic Acids (HAAs) are the second major group of DBPs after trihalomethanes. They comprise more than 25% of total halogenated DBPs in chlorinated water (Parvez et al., 2019). HAAs are formed when one or more of the hydrogens in acetic acid are replaced with halogen atoms. HAAs can be classified into three groups: monohaloacetic acids with one halogen, dihaloacetic acids with two halogens, and trihaloacetic acids with three halogens. 13 HAAs have been detected in treated water with chlorine-based disinfectants. However, only 5 HAAs known as HAA5, have been regulated by the EPA (NTP, 2018).
-66b3bd3e8b44abd62a1b8b20)
2.3. Inorganic Disinfection Byproducts
Inorganic DBPs include bromate, chlorite, and chlorate. Bromate is a byproduct of the ozonation process. If the water resource contains bromide ions, the use of ozone as a disinfectant will produce bromate through complex reactions (Aljundi, 2011). On the other hand, when chlorine dioxide is used as a disinfectant, the formation of chlorite and chlorate can occur in drinking water. Chlorine dioxide can react with organic and inorganic substances in water to form chlorate and chlorite in treated water, which can impose adverse health effects on individuals (Al-Otoum et al., 2016).
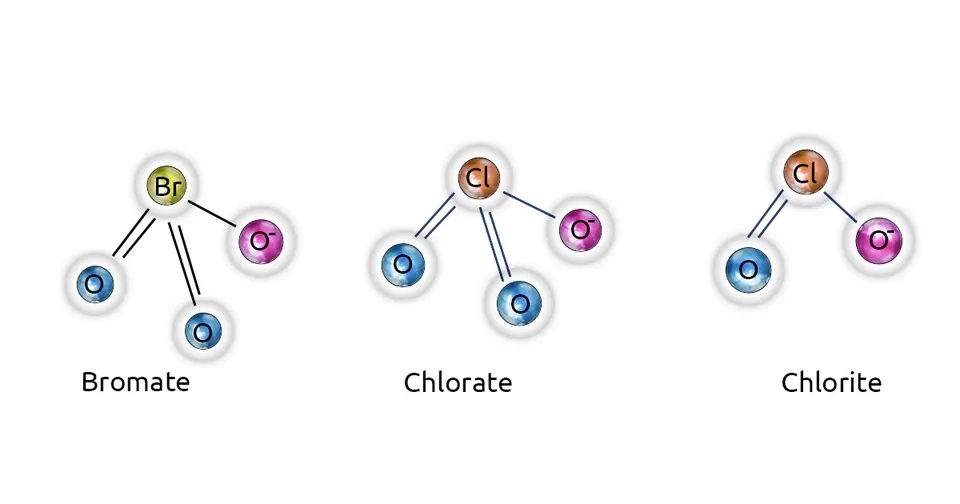
2.4. Other Halogenated DBPs
Other halogenated disinfection byproducts have been detected in addition to trihalomethanes, haloacetic acids, chlorate, chlorite, and bromate. For instance, Haloacetonitrils (HANs) form as a result of complex reactions with the presence of organic nitrogen and free chlorine. HANs form when hydrogen atoms in acetonitrile are replaced with halogen atoms (Florentin et al., 2011). HANs are classified as Nitrogenous Disinfection Byproducts (N-DBPs) along with haloacetamides. In addition to organic nitrogenous compounds in water, the use of chloramines as a disinfectant can increase the risk of N-DBP production. Although N-DBPs are usually formed in lower concentrations than THMs and HAAs, they are a great concern because of their high toxicity (Nihemaiti et al., 2016).
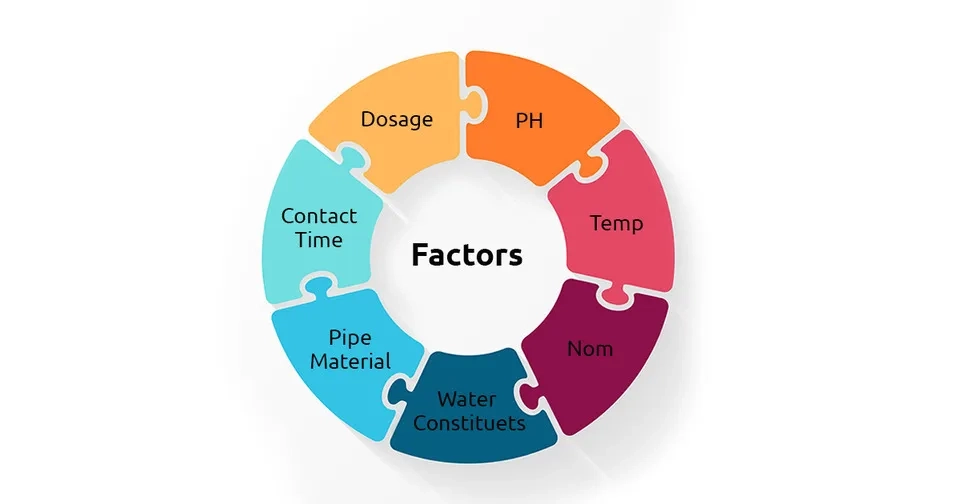
3. Factors Affecting The Formation of Disinfection Byproducts
The formation of DBPs is influenced by several factors. Some of the most important factors are disinfectant dosage, contact time, temperature, pH, and the concentration of natural organic matter. The effects of each factor are discussed in this section (Kali et al., 2021; Srivastav et al., 2020; Bond et al., 2012):
3.1. Disinfectant dosage
Studies show that a higher chlorine dosage is associated with a higher production of DBPs. Another impact of higher disinfectant dosages is the increase of intermediate DBPs, such as HANs, to a certain point. Further chlorination will oxidize the intermediate products and result in the production of THMs and HAAs as end products.
3.2. Contact time
An increase in disinfectant contact time can affect the formation of DBPs in several ways. In general, a higher contact time means the concentration of DBPs will increase since there will be a better chance of reaction between precursors and free chlorine. A longer contact time also allows the unstable byproducts to decompose into end products which results in increased formation of THMs as end products.
3.3. pH
The effect of pH on DBPs is dependent on the type of byproduct. For instance, alkaline pH (above 8) benefits the production of THMs, while HAAs are increased at acidic pH. Higher pH also promotes the hydrolysis of intermediate DBPs.
3.4. Temperature
In higher temperatures, the production of DBPs increases for several reasons. It has been observed that the concentration of NOM in surface water increases in the summer. Also, a higher temperature promotes the reaction between precursors and disinfectants. However, at very high temperatures, the level of some DBPs decreases due to their volatile nature.
3.5. Natural organic matter
Natural organic matter, as the main precursor of disinfection byproducts, is an important indicator of DBP production. The molecular features of NOM play an important role in the types of byproducts that are produced. Studies indicate that high molecular weight is associated with hydrophobicity and promotes the production of THMs and HAAs, while lower molecular weight is related to the production of brominated byproducts.
Other factors that might influence the production of disinfection byproducts are the type of pipe used in the distribution system and the constituents of water, such as bromide and iodine.
4. Adverse Effects of Disinfection Byproducts
Drinking water is not the only source of human exposure to DBPs. In fact, DBPs can enter our bodies through ingestion, inhalation, or even dermal absorption during showering or swimming (Sui et al., 2022). Some studies suggest that long-term exposure to THMs is associated with bladder cancer. Bladder cancer is the fifth most common cancer in the US, with approximately 74,000 new cases each year (Hrudey et al., 2015). A European study indicated that the risk of bladder cancer among males who consume water with THM4>50 ppb is 47% higher than that of those who consume water with THM4<5 ppb (Li and Mitch, 2017). Studies have been conducted to understand the relevance of respiratory problems and regular exposure to DBPs to pool attendance. It was indicated that chronic occupational exposure might be associated with asthma (Villanueva and Font-Ribera, 2012). Other effects, such as adverse reproductive outcomes and developmental problems, may be observed as well. However, only a few known DBPs are analyzed for their health effects, and further studies are needed to understand the effects of other DBPs (Cortéz and Marcos, 2018). It is very important to note that the disinfection process has been vital for providing safe water for communities. The World Health Organization (WHO) emphasizes that the disinfection process should not be interrupted to control the potential byproducts. In fact, the health risks associated with microbial contamination are much greater than those of the byproducts (WHO, 2022).
5. Control
There are several solutions to control the production of disinfection byproducts. Reducing the concentration of precursors is an effective way to control the production of DBPs. The main organic precursors are NOM, algal organic matter, and wastewater effluent organic matter. Various processes can be used for the removal of precursors, such as enhanced coagulation, Granular Activated Carbon (GAC), ozonation, biofiltration, and membrane filtration (Ghernaout, 2017). GAC and enhanced coagulation have been shown to be the best methods for regulating DBPs. However, if bromide is present in water resources, enhanced coagulation can promote the production of brominated DBPs. Using alternative disinfectants is another method for controlling byproducts. For instance, using chloramination instead of chlorine can reduce the formation of THMs and HAAs. But it could increase the amount of nitrogenous byproducts (Wang et al., 2015). Another solution for DBP control is post-treatment, once these byproducts are formed. Activated carbon is effective against THMs and HAAs, and biologically activated carbon can reduce the concentration of biodegradable DBPs. Air stripping is an inexpensive method for the removal of volatile substances. Thus, it can be used for volatile DBPs with less efficiency compared to adsorption (Chaukura et al., 2020). As we mentioned before, contact time and dosage play an important role in producing DBPs during the chlorination process. Therefore, optimizing the dose and time can also help decrease DBPs (Du et al., 2017).
6. Regulations
In 1998, the US EPA established the Stage 1 Disinfectants and Disinfection Byproducts Rule (DDBR), and Stage 2 DDBR was published in 2006. These two regulations were meant to help with the risk of pathogenic agents in water while reducing the production of disinfection byproducts. According to the stage 1 and stage 2 DDBRs, public water systems that use a disinfection process other than ultraviolet light must monitor the Maximum Contaminant Level (MCL) for HAA5, Total Trihalomethanes (TTHMs), bromate, and chlorite. In addition to that, Maximum Residual Disinfectant Levels (MRDLs) were established for chlorine, chloramine, and chlorine dioxide (EPA, 2020). 11 DBPs are regulated in Canada, and 5 DBPs are regulated by the European Union (EU). The Australian government and the World Health Organization (WHO) also established guidelines for 13 and 15 DBPs, respectively. However, many disinfection byproducts that remain without any regulations can be highly toxic (Richardson, 2021).
7. Conclusion
Disinfection byproducts such as trihalomethanes are formed as a result of complex reactions between disinfectants such as chlorine and organic or inorganic precursors. Hundreds of DBPs have been identified in drinking water since 1974, when the first DBPs were discovered by Rook. However, the governments only regulated a few well-known DBPs, such as THM4, HAA5, bromate, and chlorite. There are several factors that influence the formation of these unwanted chemicals, which can be used to control them. For instance, the concentration of NOM as an important precursor can be reduced with pretreatment. Post-treatment of DBPs and optimizing the dose and contact time in the disinfection process are other options that could be considered for reducing the risks. DBPs are known to be toxic for humans, and chronic exposure to them is associated with several health issues, such as bladder cancer. However, the health risk related to DBPs is insignificant compared to the risks of microbial contamination of water. Thus, the disinfection process shouldn’t be compromised.