
A method for the removal of contamination from soils that is about a century old (Yeung, 2011), but you probably haven't heard of it before!
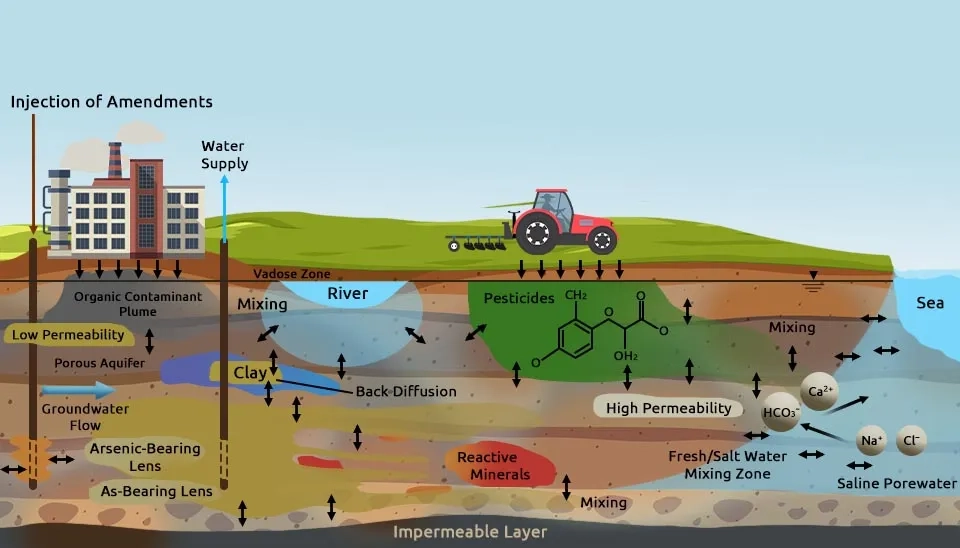
Soil pollution is a significant environmental issue that threatens ecosystem health, food security, and global sustainability. Contaminants from anthropogenic activities such as industrial processes, unsustainable agriculture, and mining infiltrate soil, water, and air, endangering human and environmental health (FAO, 2021). Among these approaches, electrokinetic remediation has emerged as a novel and effective solution for solving soil pollution, especially in challenging conditions such as low-permeability soils or heterogeneous subsurface environments. This method uses low direct current to mobilize contaminants through processes like electromigration and electroosmosis. Electrokinetic remediation can integrate and combine with other remediation technologies, such as bioremediation, to enhance its effectiveness in specific site conditions (Moghadam et al., 2016). Recent studies and advancements have highlighted electrokinetic remediation’s potential to remove different types of pollution from contaminated sites (Purkis et al., 2021). Polluted soils exhibit low strength, high water content, and complex contamination profiles, which require advanced remediation methods and approaches. Electrokinetic remediation offers sustainable solutions and pathways to mitigate soil pollution with growing research and increasing global interest, particularly from leading contributors in China, the USA, and Spain (Pandey et al., 2023).
Table. Benefits, Weaknesses, and Limitations of Electrokinetic Remediation of Contaminated Soil (Akansha et al., 2024)
Advantages and Benefits | Disadvantages and Weaknesses | Limitations |
Facilitates mobilization and removal of pollutants and ions | High energy consumption, especially in large-scale applications | Effectiveness varies depending on soil and contaminant properties |
Reduces the need for soil excavation and transportation | Requires meticulous monitoring and control to avoid unexpected outcomes | Long remediation times may be needed for deep or severe contamination |
Effective for a wide range of pollutants (heavy metals, organics, etc.) | Limited to low-permeability soils and may not suit all conditions | May not be cost-effective for small-scale or shallow contamination |
Provides a cost-efficient solution for deep-seated contamination | High upfront costs for equipment and energy use | May not address the root causes or prevent future contamination |
Minimizes environmental disturbance by avoiding soil disposal | Significant expenses for maintenance and monitoring | Might require chemicals or reagents with environmental risks |
Aids in rehabilitation of historically polluted sites | Requires technical expertise for proper operation and oversight | Ineffective for highly mobile contaminants |
Restores soil health and ecosystem integrity | Risks such as soil drying and pH imbalances could degrade soil health | Limited by contaminant solubility and non-selective for low target concentrations |
Effective for fine-grained or low-permeability soils | Relies on electricity, potentially from non-renewable sources | The corrosion of anodes requires inert materials like carbon or platinum |
Reduces costs by avoiding heavy equipment and excavation | Limited effectiveness for persistent contaminants | High voltage lowers efficiency due to heating; large rocks reduce performance. |
1. Electrokinetic Remediation
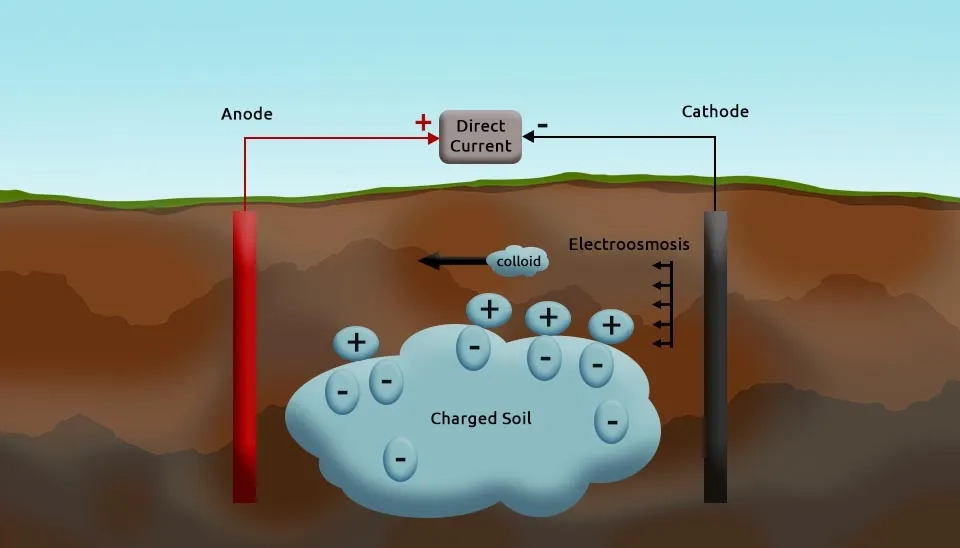
Electrokinetic remediation is an innovative method to remove contaminants from soil, sediments, sludge, and shallow groundwater by applying a direct current electric field. Electrokinetic remediation can be used in both saturated and unsaturated soils and mediums and address contamination challenges in diverse settings. Electrokinetic remediation was originally observed by Reuss in the 19th century and later elaborated by Helmholtz and Smoluchowski to identify key processes and mechanisms (Acar and Alshawabkeh, 1993). Electrokinetic remediation operates by inducing the movement of ions, charged particles, and fluids through a porous medium toward electrodes through three primary mechanisms (electroosmosis, electromigration, and electrophoresis), as well as diffusion and electrolysis. Electrokinetic remediation has been demonstrated as an effective solution in removing metals, anions, and organic pollutants such as hydrocarbons, phenols, volatile organic compounds, and heavy metals from contaminated environments. Electrokinetic remediation is also referred to as electrochemical decontamination or electro-reclamation based on the application medium (Gidudu and Chirwa, 2022).
Combining electrokinetic remediation with other technologies enhances remediation efficiency through synergistic effects. Biological processes like electro bioremediation integrate microbial degradation with electrokinetics to improve pollutant breakdown, and electro-phytoremediation combines plant-based remediation with electric fields to enhance contaminant removal. Permeable reactive barriers direct contaminants toward reactive zones for treatment. Advanced oxidation processes such as electrokinetics-enhanced in situ chemical oxidation (EK-ISCO) and Electro-Fenton utilize electrochemically generated oxidants to degrade persistent pollutants. Additionally, in situ chemical reduction can be paired with electrokinetics to facilitate the reductive degradation of contaminants. These integrations improve the contaminant removal rate and extend the applicability of remediation technologies (Gomes and Bustos, 2021).
2. Mechanisms of Electrokinetic Remediation
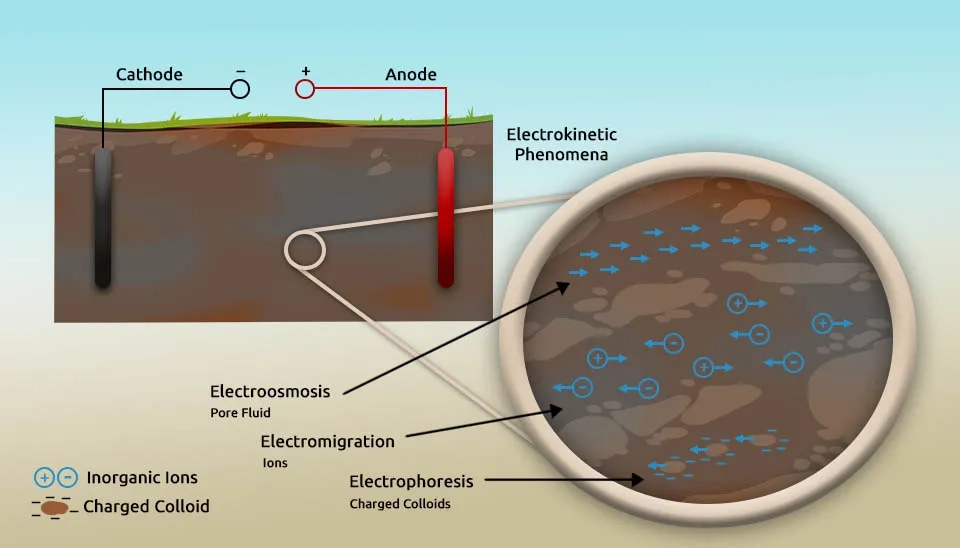
The primary mechanisms in electrokinetic remediation of contaminated soil to transport pollutants include electromigration, electroosmosis, and electrophoresis, which are explained in detail below (Han et al., 2021):
2.1. Electromigration
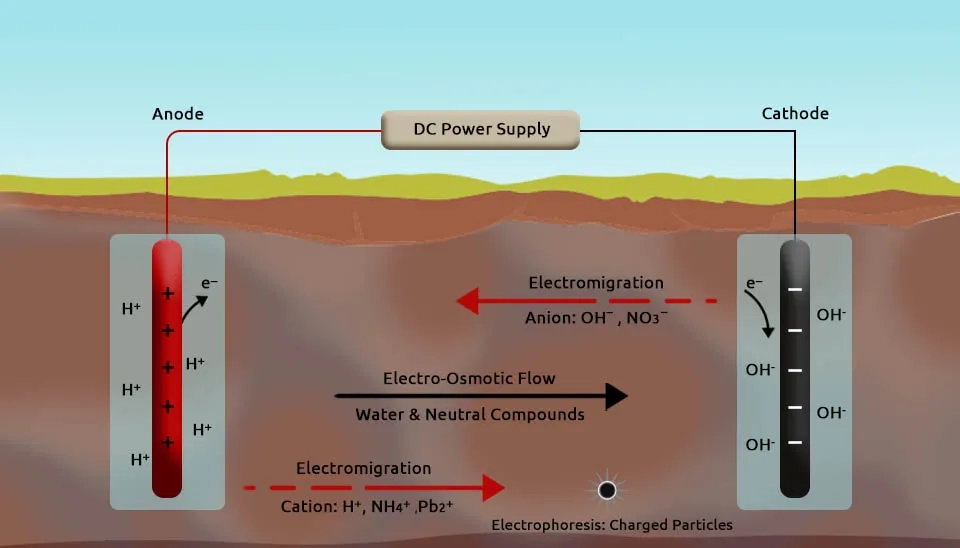
Electromigration is the movement of charged ions dissolved in an aqueous medium towards an electrode of opposite polarity under the influence of an electric field. Charged ions, such as heavy metal cations or anions, migrate through the pore water in response to electrostatic force. The anions move toward the anode and cations move towards the cathode. Electromigration is particularly effective for mobilizing heavy metal ions in pore water by facilitating their directed transport toward specific electrode zones for collection or neutralization.
2.2. Electroosmosis
Electroosmosis is the movement of pore water and its dissolved constituents in a porous medium under the influence of an electric field. This mechanism interacts with the electric field and arises due to the negatively charged surfaces of soil particles, which generate a net flow of pore fluid from the anode to the cathode. However, the direction of flow can change if the soil’s surface charge is altered through electrolysis reactions and processes. Electroosmosis transports both charged and non-charged species in the fluid and is particularly useful for mobilizing organic pollutants or other contaminants that are dissolved in pore water.
2.3. Electrophoresis
Electrophoresis is another mechanism in which charged particles, such as colloids, are transported under the influence of an electric field. Charged colloidal particles, soil organic matter, microbial cells, and other small soil particles move toward the electrode of opposite charge. Despite the contribution of electrophoresis to the movement of some contaminants, its effect is often limited due to the low mobility of charged soil particles in the electric field. Therefore, its role in electric remediation is generally less significant compared to electromigration and electroosmosis.
Furthermore, the anodic oxidation of water generates an acidic front near the anode and the reduction of water at the cathode produces an alkaline front. These processes result in the formation of H⁺ ions at the anode and OH⁻ ions at the cathode, which participate in electromigration. These species alter the pH of the soil and cause localized changes in soil chemistry. pH changes can trigger a variety of chemical reactions, such as the dissolution of salts and minerals, which enhance the mobilization of pollutants in the soil. These changes can also promote the precipitation of certain compounds, trap pollutants, and impede their release (Vocciante et al., 2021).
3. Types of Contaminants Treated with Electrokinetic Remediation
Electrokinetic remediation effectively removes organic as well as inorganic contaminants such as heavy metals, radioactive elements, and salts from soil, which address risks and challenges to human health and the environment. Electrokinetic remediation is suitable for low-permeability soils to transport contaminants, with applications in saline-alkaline soil and municipal sludge treatment (Wen et al., 2021). In the following, two major types of contaminants treated with electrokinetics are discussed thoroughly:
3.1. Electrokinetic Remediation for the Removal of Heavy Metals
Electrokinetic remediation has proven to be an effective technology for the removal of heavy metals like cadmium (Cd), copper (Cu), lead (Pb), zinc (Zn), chromium (Cr), mercury (Hg), selenium (Se), and arsenic (As) from contaminated soils, especially low-permeability soils that traditional methods are challenging. Heavy metals are bioaccumulative and difficult to degrade and pose significant environmental and health risks (Sun et al., 2023). Electrokinetic remediation utilizes electrical currents to mobilize and remove metal ions, but its efficacy can be limited by factors such as weak metal solubilization, energy consumption, and the accumulation of metals near electrodes rather than full extraction. Using tailored electrolytes and ion exchange membranes can improve metal desorption and pH control, enhancing metal removal efficiency. Innovative solutions, including the use of renewable energy sources like solar power and microbial fuel, can further reduce energy costs and improve sustainability. Coupling electrokinetic remediation with other technologies such as chemical stabilization or bioremediation can further improve heavy metal removal (Wang et al., 2021).
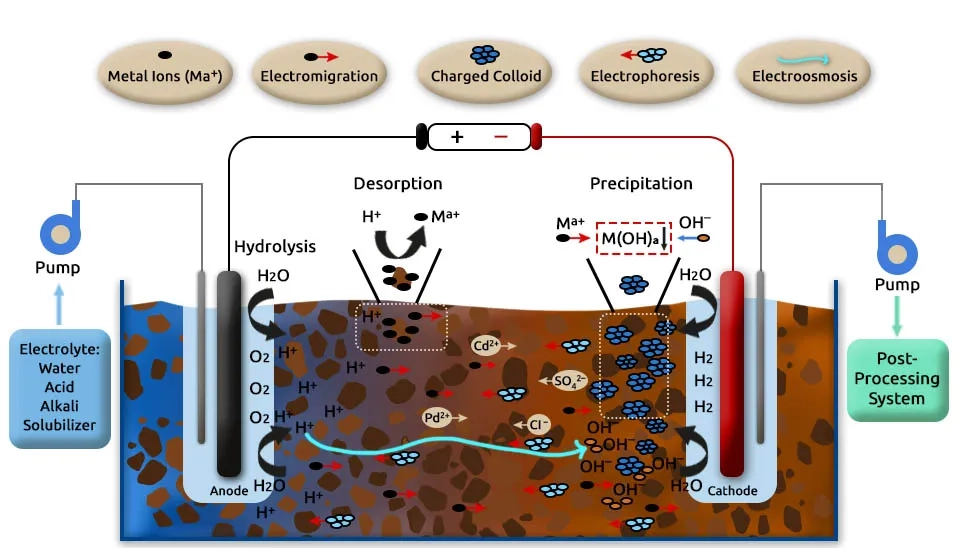
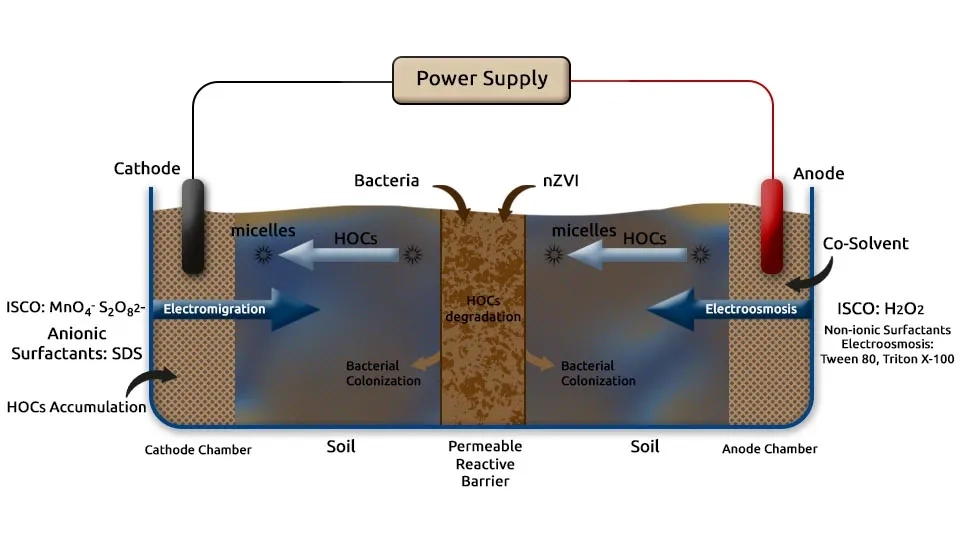
3.2. Electrokinetic Remediation for the Removal of Organic Contaminants
Electrokinetic remediation has been used as a promising solution for the removal of organic contaminants in soil, particularly hydrophobic organic compounds (HOCs) and other persistent pollutants. These compounds are challenging to remediate due to their low solubility and lack of charge, which limits their remediation via electromigration and electroosmosis. Consequently, agents such as surfactants, co-solvents, and nanoparticles are introduced to enhance contaminant solubility and promote in situ degradation. Electrokinetic remediation is also often combined with complementary technologies like in situ chemical oxidation, bioremediation, and phytoremediation to achieve efficiency in contaminant removal. These hybrid approaches enable the decomposition of organic contaminants directly within the soil matrix through the delivery of chemical agents or biological enhancers. Further improvements depend on optimizing these combinations and integrating facilitating agents to overcome site challenges (Cameselle and Gouveia, 2018).
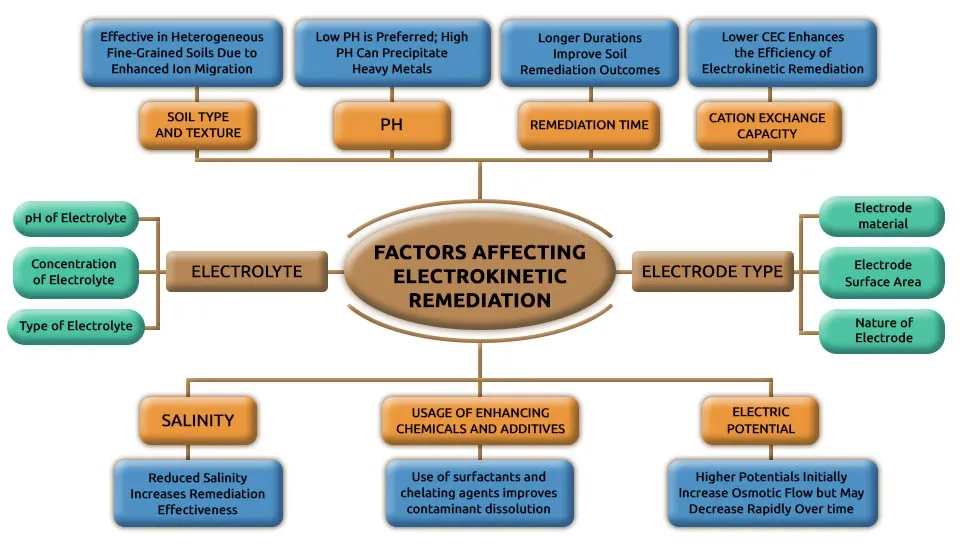
4. Factors Affecting Electrokinetic Remediation
The efficiency of electrokinetic remediation depends on various soil and operational factors. Soil type and structure significantly influence contaminants mobility. For example, fine-grained soils like clay are more amenable due to their surface charge and pore water content. The pH as well as pH gradients that are created by electrolysis reactions, also play a critical role by changing soil chemistry, enhancing contaminant dissolution, and causing precipitation near electrodes. The type and concentration of contaminants, as well as the conductivity of the soil pore water, impact their migration and removal rates. Applied current density and soil saturation further affect ion and fluid movement and determine the contamination transport rate. Soil conditioning with surfactants or chemical reagents can improve remediation by increasing the mobility of contaminants. For example, electroplating, precipitation, or complexation can remove contaminants by migration to electrodes. Moreover, field conditions such as organic content, water content, and contamination distribution must be considered to optimize the process. These factors should be considered to achieve successful removal of contaminants in soil by electrokinetic remediation (Virkutyte et al., 2002).
5. Computational Modeling of Electrokinetic Remediation
The modeling approach for multidimensional electrokinetic transport integrates process-based analysis to capture the complex interplay between physical, electrostatic, and biogeochemical processes in saturated porous media through an electrokinetic mechanism. The modeling framework and governing equations enable a deeper understanding of the mechanisms driving the remediation process and support the optimization of this technique (Sprocati et al., 2020), which are discussed below:
5.1. Governing Equations of Electrokinetic Remediation
Electrokinetic remediation and transport of charged species in a porous medium is governed by the principles of diffusion, electromigration, and advection. The electrochemical potential μi for an ion i is defined as (Sprocati et al., 2019):
μi = μi0 + RT ln(ai) + zi FΦ
Where μi0 is the standard-state electrochemical potential, ai is the ion activity, zi is the ion’s valence, R is the ideal gas constant, T is the temperature, F is Faraday’s constant, and Φ is the electrostatic potential. The spatial gradient of this potential that drives the flu of the i-th species is described by the Nernst-Planck equation according to the following equation:
Ji = - Di ∇ci - Di ∇ci ln(γi ) - DiziFci∇Φ/RT
Where Ji represents the total ionic flux, Di is the diffusion coefficient, which is adjusted for tortuosity in porous media, ci is the concentration, γi is the activity coefficient, and ∇Φ is the gradient of the electric potential. These three terms in the above equation correspond to diffusive flux, activity gradient flux, and migration flux, respectively. Under flow-through conditions, Di is replaced by the hydrodynamic dispersion coefficient to show advective contribution. Furthermore, a material balance for each ionic species leads to the governing multicomponent transport equation as described below:
∂(nci)/∂t+∇⋅Ji=ri
Where n is the porosity, Ji is the total flux, ri is related to reactive sources or sinks. To ensure charge neutrality within the medium, the Possion equation relates the electric charge density to the electrostatic potential:
∇2Φ=ρe/ϵ
Where ϵ is the permittivity of the medium, and ρe is the electric charge density. These equations describe the coupled transport phenomena for electrokinetic transport and remediation.
5.2. Numerical Modeling of Electrokinetic Remediation
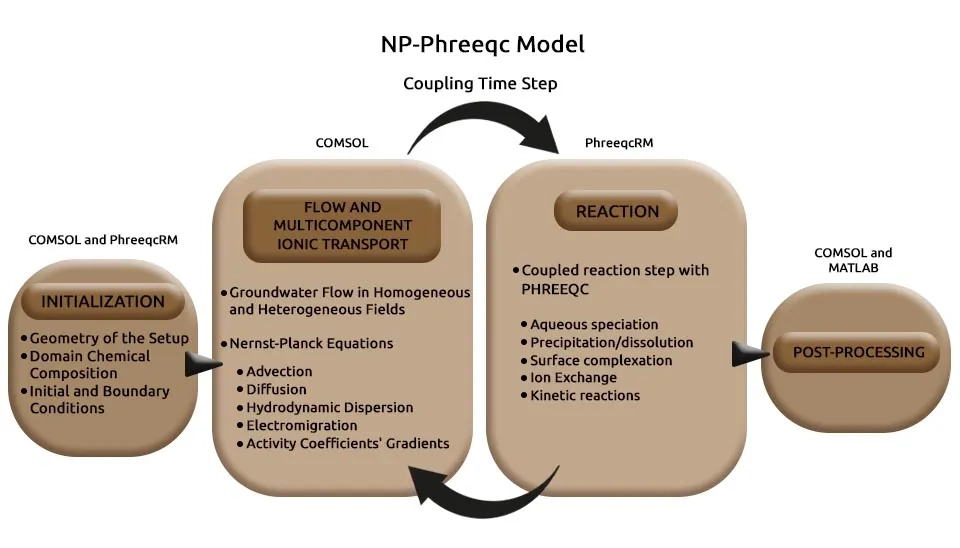
Coupling solute transport models with geochemical simulators has enhanced the understanding and numerical modeling of subsurface systems such as shallow groundwater by allowing the modeling of complex interactions between transport processes and geochemical and biogeochemical reactions. Various reactive transport simulators have been developed to describe these processes, including CrunchFlow, Geochemist’s Workbench, MIN3P, PHT3D, TOUGHREACT, and PHAST, each tailored for specific applications such as groundwater systems or non-isothermal and multiphase flow (Rolle et al., 2018). Furthermore, several computational fluid dynamic models have been utilized to simulate fluid flow and transport, including COMSOL (Rolle and Sprocati; Masi et al., 2019; Masi et al., 2017; López-Vizcaíno et al., 2021), OpenFOAM (Barnett et al., 2023; Pimenta & Alves, 2019; Pimenta & Alves, 2018; Pham & Van, 2022), and FEniCS (Xie & Lu, 2020; Ying et al., 2021; Linga et al., 2019; Farooqi et al., 2019). Computational fluid dynamic models such as OpenFOAM and FEniCS are open source, and several boundary conditions (conductive, insulating, inlet/outlet, periodic, permeable membrane, and reactive boundaries) are tested to simulate advective, diffusive, and electrostatic forces of an ionic solution (Barnett et al., 2023).
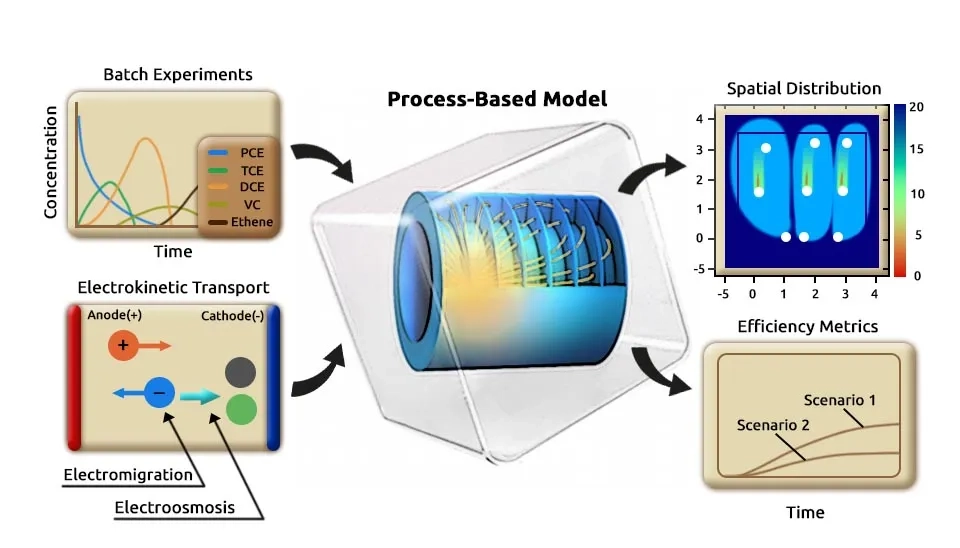
Among computational fluid dynamic tools, COMSOL Multiphysics has been used to simulate electrokinetic transport processes in porous media. COMSOL Multiphysics is able to couple with geochemical simulators such as PhreeqcRM to model conservative and reactive transport in multidimensional saturated porous media. This approach is based on the Nernst-Planck equation and allows for the description of physical displacement, coulombic interactions, and a wide range of geochemical reactions. The coupling framework utilizes LiveLink of MATLAB to facilitate the connection between COMSOL and PhreeqcRM software. In this framework, flow in homogeneous and heterogeneous saturated porous media and Nernst-Planck equations are solved in COMSOL, and geochemical reactions are solved in PhreeqcRM. Integrating these modules into a multi-purpose simulation platform like COMSOL combines the finite element methods with the robust capabilities of PhreeqcRM as a reaction engine (Rolle et al., 2018; Sprocati and Rolle, 2021).
6. Advantages and Limitations of Electrokinetic Remediation
Here, the advantages and limitations of electrokinetic remediation of contaminated soil and porous medium are discussed in detail:
6.1. Advantages of Electrokinetic Remediation
Electrokinetic remediation is a unique and versatile method to remove soil and porous medium contamination. It is particularly effective for treating low-permeability soils and fine-grained sediments where traditional in situ methods are not highly suitable. Electrokinetic remediation operates in situ and requires no excavation or large equipment, unlike other traditional methods, thus reducing costs and minimizing disruption to the existing landscape (Pham and Sillanpää, 2015). Electrokinetic remediation can remove charged metal ions via electromigration and non-charged pollutants through electroosmotic glow by leveraging directed flow throughout the electric field, which makes it useful for a wide range of contaminants. It is also an environmentally friendly approach without introducing harmful chemicals into the treated soil and porous media (Sun et al., 2023). Furthermore, electrokinetic remediation is adaptable and can enhance other remediation techniques like bioremediation and chemical oxidation. This method is cost-effective compared to ex-situ methods and safety in operation, especially for urban and sensitive areas where visual impact and operational safety are important (Akansha et al., 2024).
6.2. Limitations of Electrokinetic Remediation
Electrokinetic remediation faces several limitations and challenges that restrict its wide implementation, which should be considered in future studies. One of its limitations is its limited efficiency in soils with low contaminant concentrations or high non-target concentrations since the process is non-selective and dependent on contaminant solubility and desorption. Furthermore, electrokinetic remediation’s efficiency decreases with larger rocks or non-fine and coarse materials in the soils and requires close electrode spacing to address transport limitations. Corrosion of electrodes in an acidic environment makes it necessary to use expensive inert materials like graphite or platinum, which increases the complexity and expense of the process (Pham and Sillanpää, 2015). Additionally, electrokinetic remediation requires high energy, especially when extracting adsorbed contaminants like heavy metals, which increases costs and treatment duration. Acidification during electrokinetic remediation can alter soil geochemistry and microstructure, which negatively affects long-term soil health (Alshawabkeh, 2009). These limitations and challenges in electrokinetic remediation highlight the need for careful planning and optimization in their applications.
7. Conclusion
Electrokinetic remediation is a promising and innovative technology for the removal of contaminants in soils, including heavy metals and organic pollutants, through mechanisms including electromigration, electroosmosis, and electrophoresis. This method uses the application of an electric field to mobilize and transport contaminants, especially in fine-grained soils like clay, where traditional remediation methods are less efficient. Electrokinetic remediation has demonstrated significant performance in solving challenges posed by heavy metals, such as cadmium and lead, as well as persistent hydrophobic organic compounds. Advances in computational modeling, such as numerical models and governing equations, have enhanced our understanding of electrokinetic processes. Factors such as soil type, pH gradients, contaminant types and properties, and operational parameters play a critical role in electrokinetic remediation’s success. Furthermore, hybrid and combined approaches combining electrokinetic remediation with chemical stabilization, bioremediation, or the use of facilitating agents like surfactants and nanoparticles provide effective solutions for overcoming challenges. In general, electrokinetic remediation is cost-effective and suitable for diverse contamination scenarios; however, its limitations include high energy demands, electrode corrosion, and localized accumulation of contaminants. Future research and technological advancements are essential for improving the efficiency of electrokinetic remediation and expanding its application across different environments.

