
Per-and polyfluoroalkyl substances (PFAS) are contaminants of emerging concern, and the presence of these synthetic chemicals in drinking water calls for immediate actions to protect communities. In the Bipartisan Infrastructure Law, an investment of $10 billion is considered for the next 5 years to help communities with PFAS contamination in their drinking water. The Environmental Protection Agency (EPA) is taking action and proposing the first national drinking water standard for six PFAS compounds (EPA, 2023). Human exposure to PFAS can have many negative effects, such as immune dysfunction, kidney disease, liver damage, and thyroid dysfunction (Jha et al., 2021). The conventional water treatment process is not effective against PFAS substances (Franke et al., 2019). Therefore, the development of effective PFAS removal technologies in contaminated water is urgent to reduce the health impacts of these harmful chemicals. This article aims to discuss various methods that are currently available for removing PFAS from our water supplies. Granular Activated Carbon (GAC), ion exchange, and high-pressure membrane technology are some of the known practical methods. We will explain each method individually, along with its efficiency and cost-effectiveness. The following table is a comparison between three practical methods that are used as PFAS removal technologies (AWWA, 2019)
Table. A comparison between practical methods used for removing PFAS
PFAS Removal Method | Advantages | Disadvantages |
Granular Activated Carbon (GAC) | -High removal efficiency
-It’s widely used due to lower costs compared to the other two methods | -Not very effective against short-chain PFAS -The presence of Natural organic matter will cause competitive Adsorption -Requires regeneration which adds to operational costs |
Anion Exchange Resin (AER) | -Unlike GAC, it can be used for short-chain PFAS as well as long-chain -Lower Empty Bed Contact Time (EBCT) compared to GAC | -higher in costs and especially regeneration is more expensive |
Membrane Technology | - Great rejection rate for a wide range of PFAS contaminants | - concentrated waste stream - higher costs compared to GAC and AER |
1. Three Main PFAS Removal Technologies in Water Treatment
1.1. Granular Activated Carbon (GAC)
Granular Activated Carbon (GAC) is an adsorption technology, and it is one of the most studied technologies applied for treating PFAS in water (Burkhardt et al., 2022). GAC is a porous carbonaceous material with a high surface area to adsorb pollutants. PFAS adsorption is related to the hydrophobicity of these chemicals. Long-chain PFAS are more hydrophobic, and GAC can effectively adsorb them. However, this method does not work for eliminating short-chain PFAS compounds because of their hydrophilicity features (Zeng et al., 2020; Lei et al., 2023). An important parameter in designing granular activated carbon is Empty Bed Contact Time (EBCT) which is the contact time between the media and water. A typical EBCT of 10 to 20 minutes is recommended for GAC (Murray et al., 2021; Patterson et al., 2019).
GAC requires regeneration to remove the adsorbed PFAS from the media and restore its capacity. However, regenerated GAC is less effective, and the regeneration process is costly (Wanninayake, 2021). Another limitation of activated carbon is the presence of competitive pollutants such as Natural Organic Matter (NOM) which can reduce the adsorption of PFAS substances. Nevertheless, GAC is widely used for PFAS removal in drinking water, and an efficiency of 99% is reported for eliminating Perfluorooctane Sulfonate (PFOS) (Miliato et al., 2021).

1.2. Anion Exchange Resins (AER)
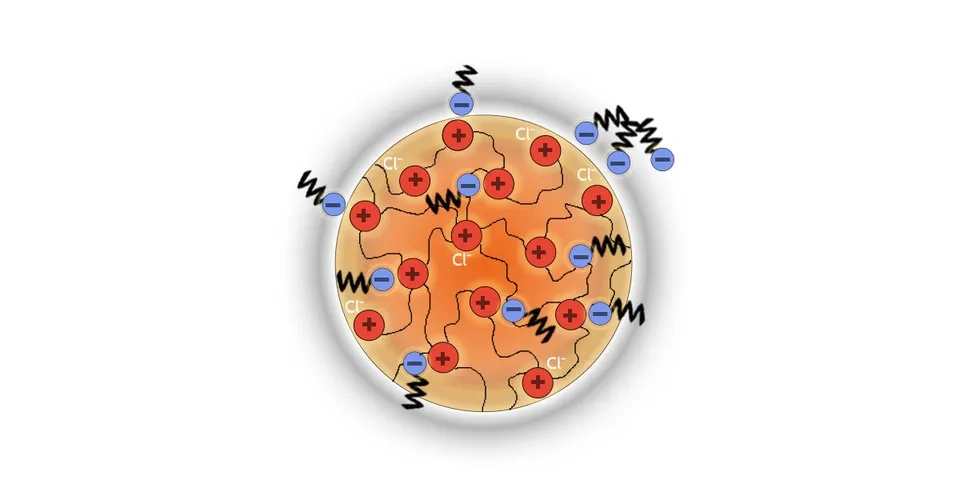
Anion Exchange Resin (AER) is another widely used method for PFAS removal that removes PFAS through adsorption and ion exchange. The PFAS structure consists of a hydrophobic tail and a negatively charged head. The hydrophobic tail of the PFAS is attracted to the hydrophobic backbone of the resin, while the positively charged surface of the resin can exchange chloride with the negative head of the PFAS (Vu and Wu, 2020; Woodard et al., 2017).
Unlike GAC, anion exchange resins can be effective against short-chain PFAS and they are higher in adsorption capacity (Crone et al., 2019). Compared to GAC, AER is a more expensive process, and regeneration costs are higher. However, it needs less empty bed contact time. Because of the higher costs, it is recommended to consider AER as a second option if GAC does not show effective results (Verma et al., 2021).
1.3. Membrane Technology
High-pressure membrane filters such as Reverse Osmosis filters (RO) and NanoFilters (NF) can be used for removing PFAS from water. RO filters have an excellent rejection rate for both long-chain and short-chain PFAS. In fact, RO filters have a removal efficiency of above 99% for PFOS and Perfluorooctanoic Acid (PFOA). Nanofiltration technology shows less efficiency for short-chain PFAS since they can pass through membrane pores more easily (Jin et al., 2021; Lee et al., 2022). A major drawback of membrane filtration technology is it's relatively higher cost compared to GAC and AER. Another limitation is the concentrate stream that contains PFAS and needs to be treated and safely managed (Westreich et al., 2018).
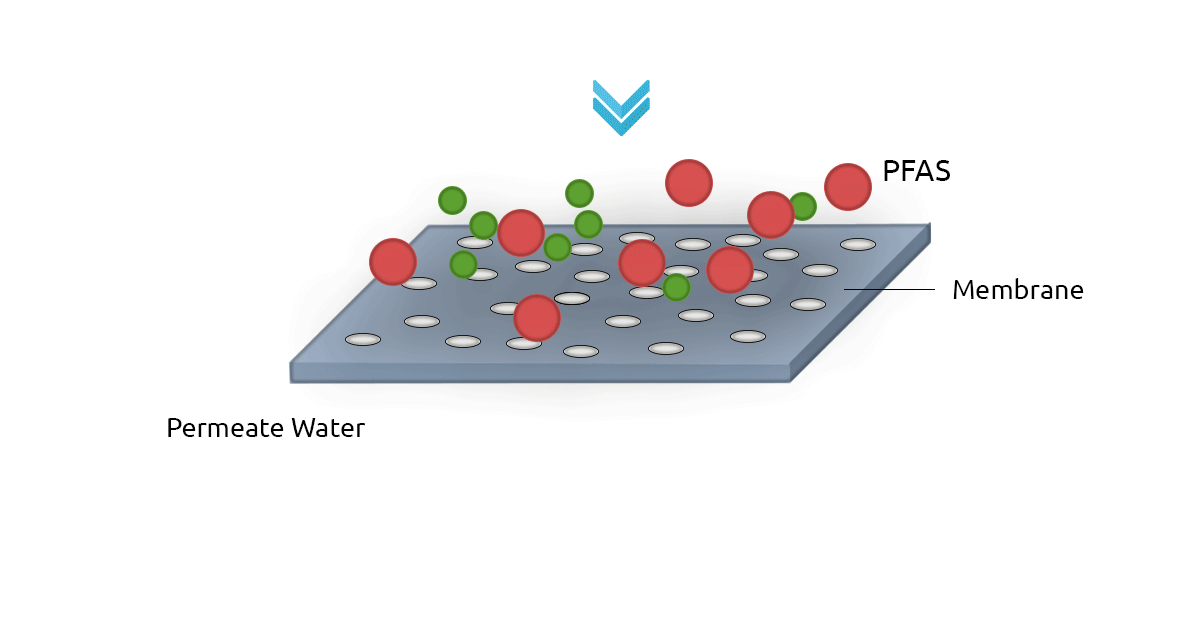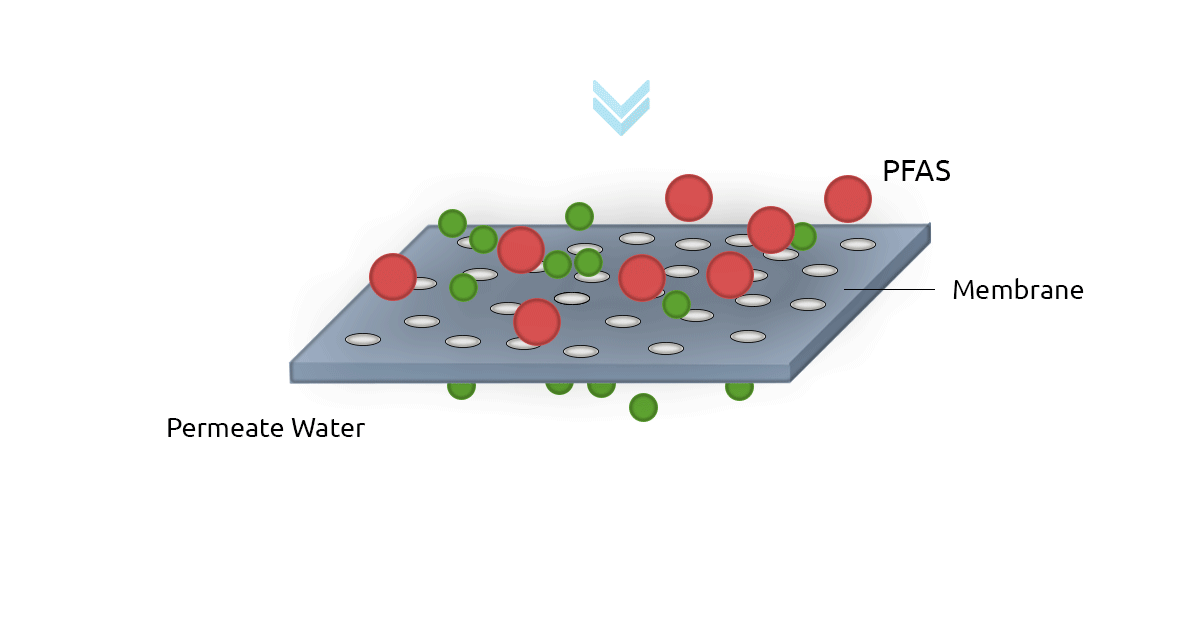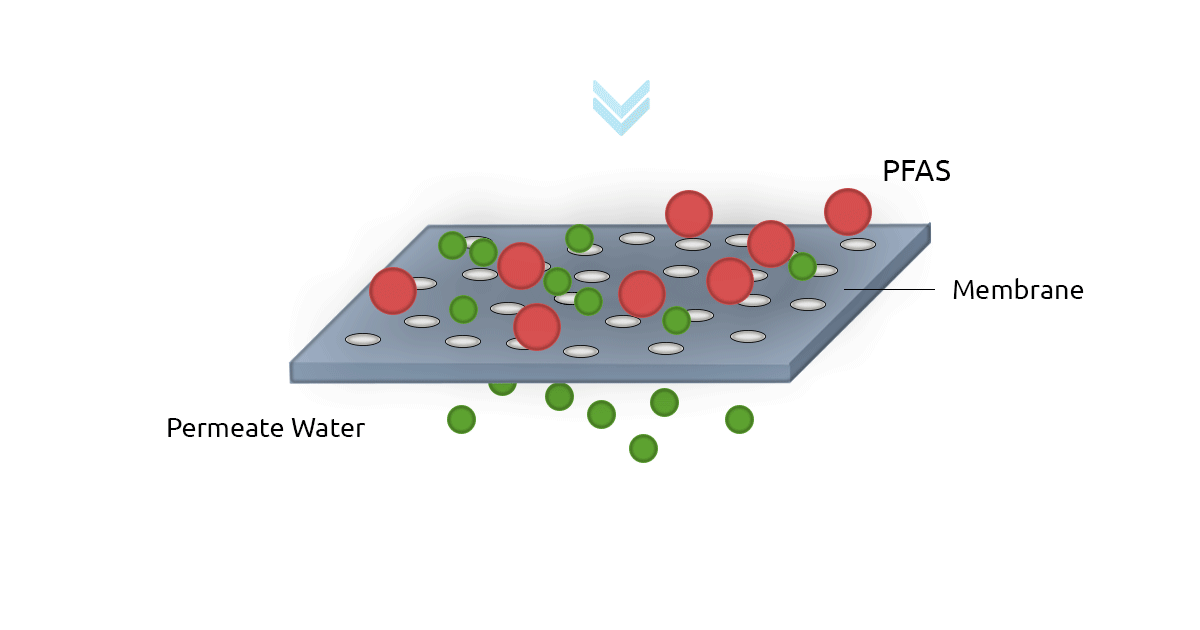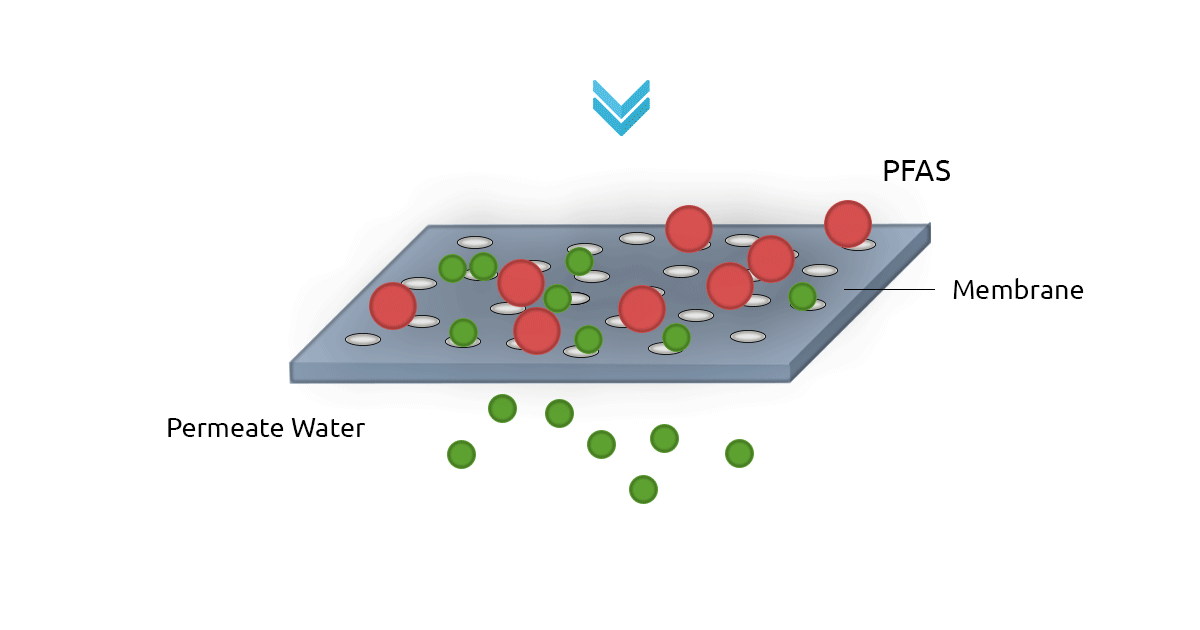
2. Other Treatment Methods
In addition to the practical methods that were mentioned earlier, researchers are studying other treatment technologies for PFAS removal in drinking water. Some of them are addressed below.
2.1. Plasma Technology
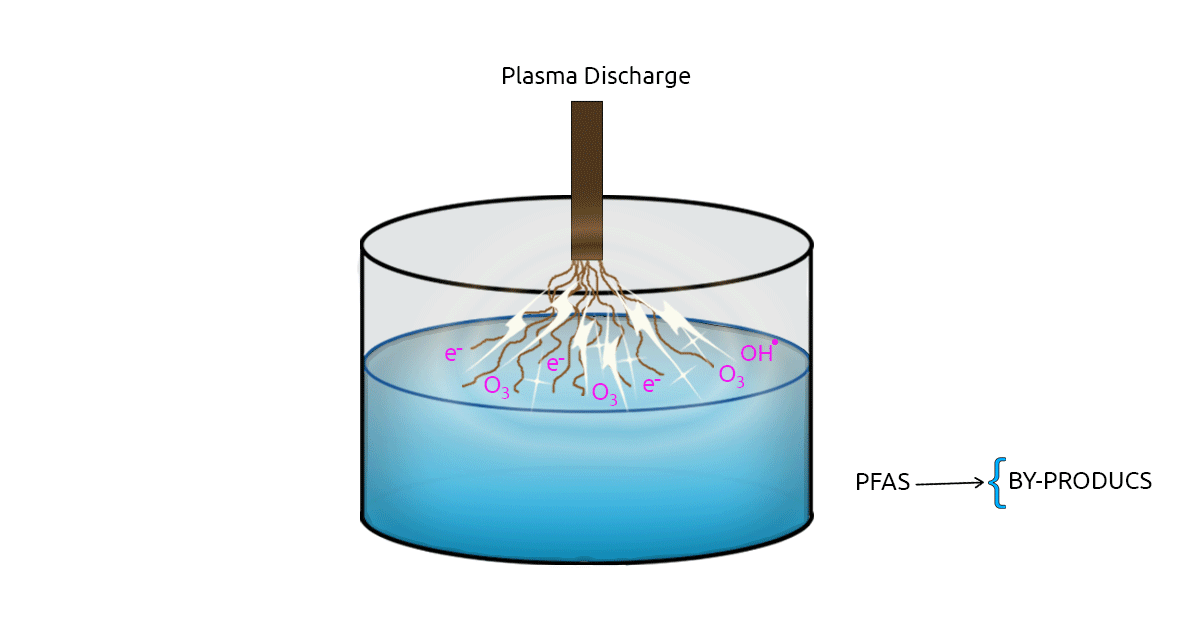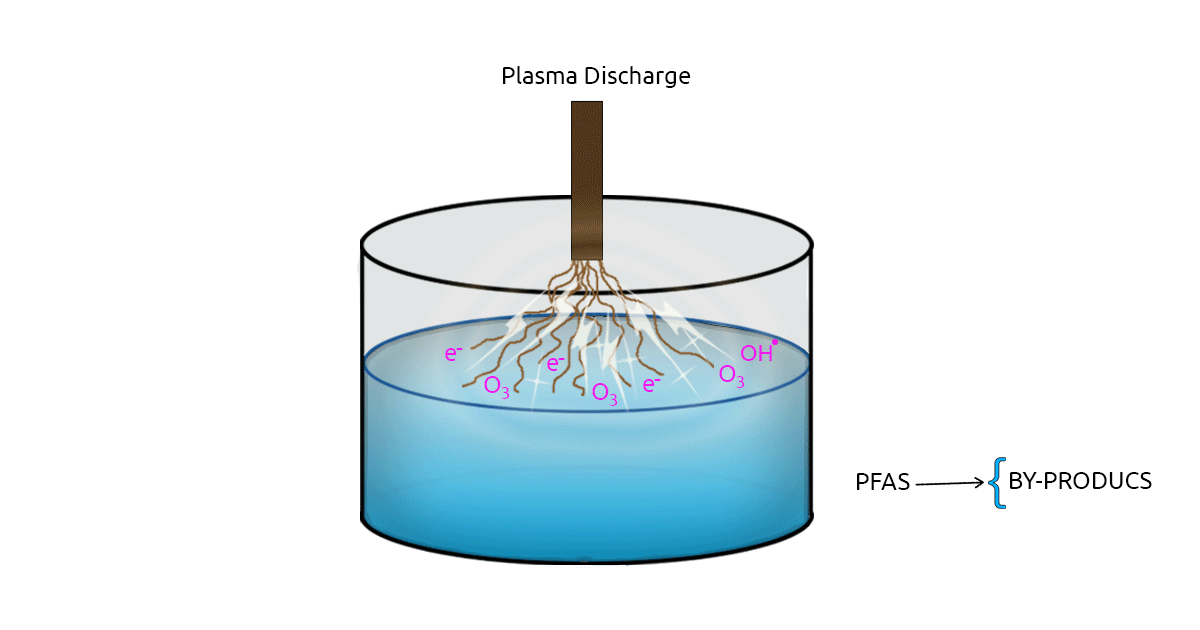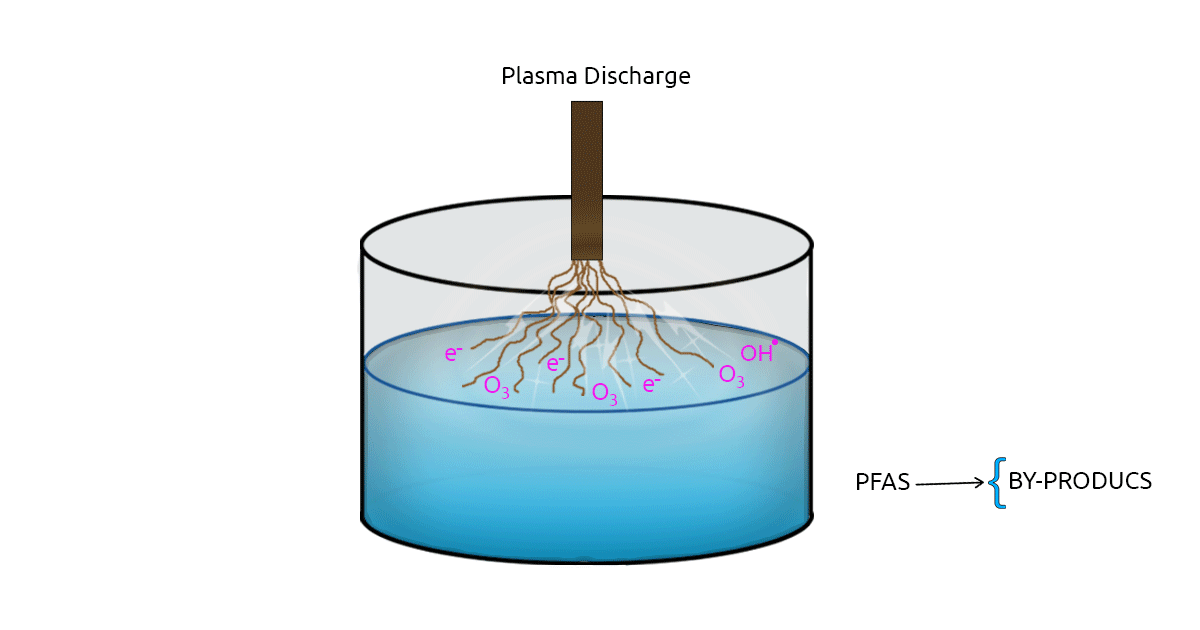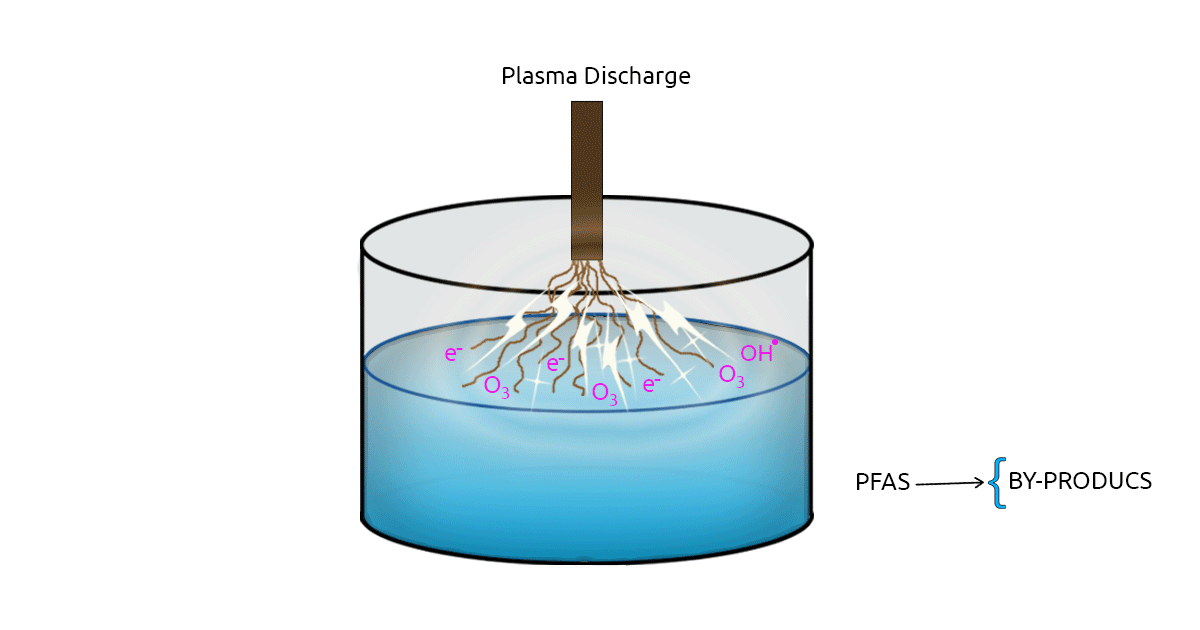
Many Advanced Oxidation Processes (AOPs) are studied in water treatment for removing contaminants of emerging concern. Plasma technology, as an advanced oxidation process, has drawn the attention of many researchers as a PFAS removal method. Plasma is an electrically charged gas. In plasma-based methods, electrical discharge between two electrodes results in the formation of highly reactive radicals, ozone, and free electrons. There are two types of Plasma-based methods: Thermal plasma and Non-Thermal Plasma (NTP). Non-thermal plasma is favored in water treatment due to its lower energy consumption (Palma et al., 2021; Nzeribe et al., 2019). The effectiveness of NTP in removing PFOA from distilled water was evaluated in a study. In this study, NTP treatment was able to remove 90% of the PFOA concentration in 60 minutes (Khan et al., 2020).
2.2. Bioremediation
Treating PFAS substances biologically is very challenging due to the persistent nature of these chemicals. Biodegradation of these compounds requires a significant amount of time, on the scale of weeks and months. There are only a few enzymes discovered so far to be effective on PFAS, and they are only working on certain PFAS compounds. The main reason for these challenges is that microorganisms were not exposed to synthetic fluorinated compounds for enough time to evolve. However, with further studies on this subject, these challenges may be overcome since bioremediation is an environment-friendly and cost-effective method (Wackett et al., 2021).
2.3. Electrocoagulation
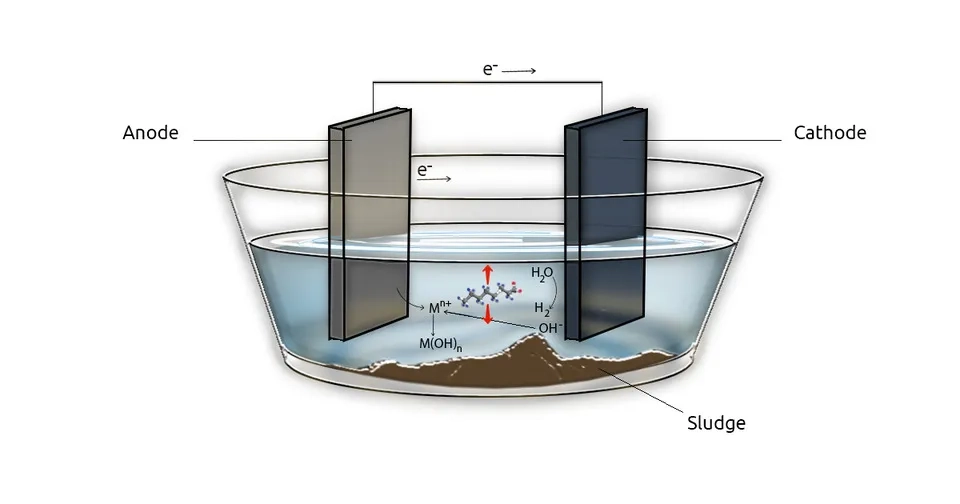
Electrocoagulation (EC) consists of two electrodes, submerged in a conductive solution, that are attached to a power supply to apply direct current. EC can eliminate PFAS substances in two ways: non-destructive separation or destructive. Compared to other destructive methods like advanced oxidation, EC consumes less energy. Through non-destructive electrocoagulation, metal hydroxide flocs are created and PFAS substances are removed by sorption(Mao et al., 2023).
3. Conclusion
The application of PFAS removal methods is challenging due to the nature of fluorinated compounds and the strong carbon-fluorine bonds. Currently, three effective processes are used, including activated carbon, anion exchange, and membrane technology. Each of them has its own advantages and disadvantages, and the choice of process depends on various circumstances, including the type of PFAS. Removal of short-chain PFAS can be challenging for some methods, like GAC. Apart from these three methods, researchers are studying other potential treatment options. Further development of these technologies can lead to reduced costs in the treatment process and increased removal efficiency.