
Groundwater or GroundSaline?! Although we expected to reach fresh water by pumping from groundwater until decades ago, what do you think about the future? Several factors have made groundwater salinity a significant threat to our future. The threat of groundwater salinity is not limited to a specific geographical location, covering the entire earth, and is becoming more acute day by day. Take the western United States as an example. In New Mexico, approximately 75% of groundwater is unusable without treatment (USGS, 2018). Hence, it is vital to take a closer look at groundwater salinity.
Even though 98% of freshwater is in groundwater, population growth and industrial and agricultural development add 1 to 3% annually to the amount of groundwater withdrawal. Groundwater depletion and contamination have many quantitative and qualitative consequences for the ecosystems in the groundwater and related ecosystems. On the other hand, climate change and other factors intensify the effects of groundwater depletion and contamination, which requires groundwater monitoring and management to increase the sustainability of groundwater resources in the long term (Gude, 2018; Taylor et al., 2013). One of the challenges related to the quality and quantity of groundwater is its salinity.
Freshwater salinization is a significant challenge related to water quality that affects surface water and groundwater sources. Groundwater sources are valuable sources of fresh water supply, which have faced the salinization crisis in recent years due to anthropogenic and natural factors in groundwater salinization. The significant negative effects of groundwater salinization on a wide range of ecosystems and human life have caused increasing attention to groundwater salinity problems (Thorslund and Vliet, 2020).
1. Definition of Groundwater Salinity
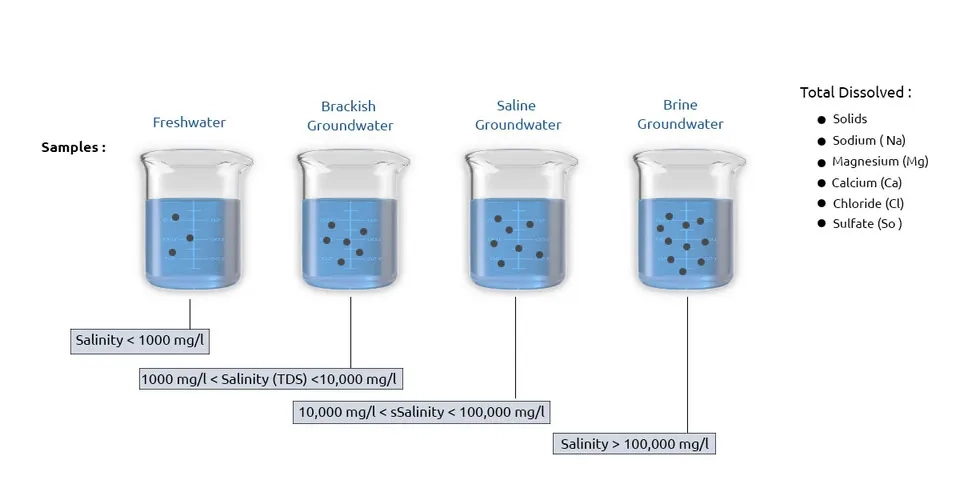
Groundwater salinization is one of the most important challenges worldwide, especially in water-scarce areas. Here, salinity refers to Total Dissolved Solids (TDS), which is reported in milligrams per liter (mg/L). Sodium (Na), magnesium (Mg), calcium (Ca), sulfate (SO4), and chloride (Cl) ions make up the TDS and salinity in groundwater, while the majority of seawater salinity is related to sodium (Na). and chloride (Cl). It is worth mentioning that in some cases, groundwater salinity refers to the concentration of chloride ions, and in some cases, the Electrical Conductivity (EC) in terms of microS/cm is used to measure groundwater salinity (Salem and Osman, 2017; Boluda-Botella et al., 2008).
According to the classification provided by Freeze and Cherry (1979), groundwater salinity is divided into four categories: 1) freshwater with salinity less than 1000 mg/L, 2) brackish groundwater with salinity between 1000 and 10000 mg/L, 3) Saline groundwater with salinity between 10,000 and 100,000 mg/L, and 4) ebrin groundwater with salinity over 100,000 mg/L. The World Health Organization (WHO) and the Environmental Protection Agency (EPA) have defined a chloride concentration equal to 250 mg/L and a chloride and TDS concentration between 250 mg/L and 500 mg/L as the allowable limit of groundwater salinity, respectively (WHO, 2011; EPA, 2011).
2. Causes of Salinity in Groundwater
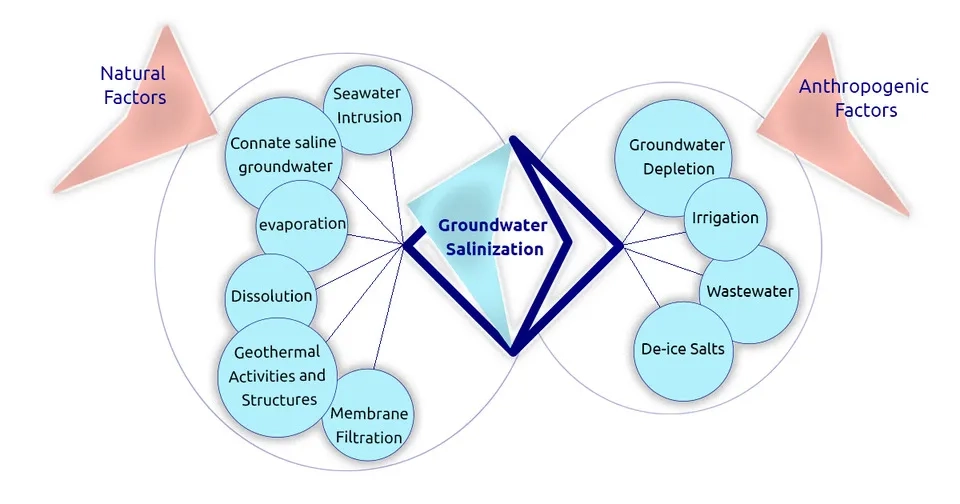
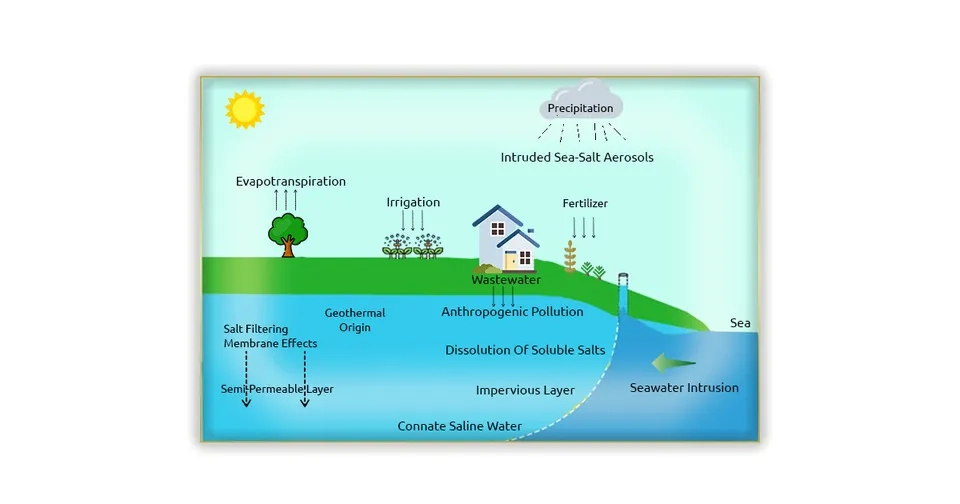
Several factors cause the salinity in groundwater, and previous studies have presented divisions to separate these factors.Frank van Weert et al. (2009)divided the origins of groundwater salinization into four categories:1) Marine origin, 2) Terrestrial and natural origin, 3) Terrestrial and anthropogenic origin, and 4) Mixed origin.Li et al. (2020) also divided the causes of salinity in groundwater into two categories: 1) Natural origin and 2) Anthropogenic pollution. Natural factors can affect groundwater salinity on a large spatial scale, while the effects of anthropogenic factors are generally regional and local. Here, we have separated the causes of salinity in groundwater into two categories, anthropogenic and natural factors in groundwater salinization, and we will examine each of them in detail in the following.
2.1. Natural Factors (Marine and Terrestrial Origin)
Natural factors that have terrestrial and marine origin can lead to the salinization of groundwater in the short and long term on a regional and global scale. In the following, we will focus on important natural factors:
2.1.1. Seawater Intrusion
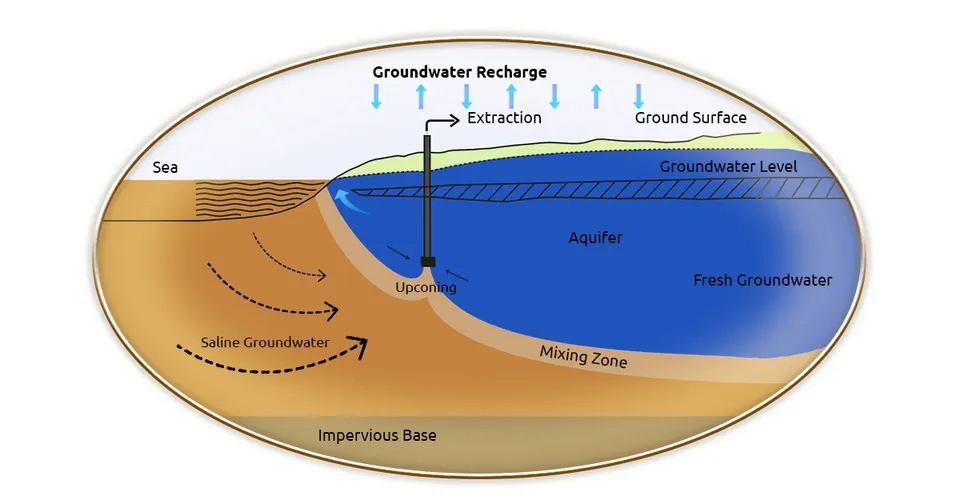
Seawater intrusion is a process in which seawater is transferred to freshwater (groundwater) in coastal aquifers and leads to the salinization of groundwater. The high salinity and minerals of groundwater and the presence of a connection between seawater and groundwater cause high pressure and denser seawater to penetrate into underground aquifers. The following factors can directly or indirectly cause groundwater salinization through seawater intrusion (transfer of seawater to coastal aquifers) which, depending on their nature, have a secondary human or natural origin (Grant and Tom, 2012; Tosaki et al., 2017).
Sea level rise
Reduction of freshwater head caused by excessive consumption of groundwater
Drainage networks and canals
Tidal fluctuations
Climate change
Marine transgressions (Flooding of coastal lowlands during periods of marine transgressions and downward seawater flow due to density differences)
Incidental Flood (Drowning of low-lying coastal plains by seawater, intrusion of seawater into shallow coastal aquifers)
Seawater sprays (Absorption of salt particles originating from the sea by rain and transfer to the groundwater feeding waters and finally groundwater salinization).
2.1.2. Connate Saline Groundwater
Connate saline groundwater is formed from the formation of marine sediment and stored in the interstices along with the rock matrix. Although the transfer of connate saline groundwater is slow, connate water can be saltier than seawater. High salinity and the presence of mineral-ionic compounds in connate cause it to be denser than seawater (Yager et al., 2017).
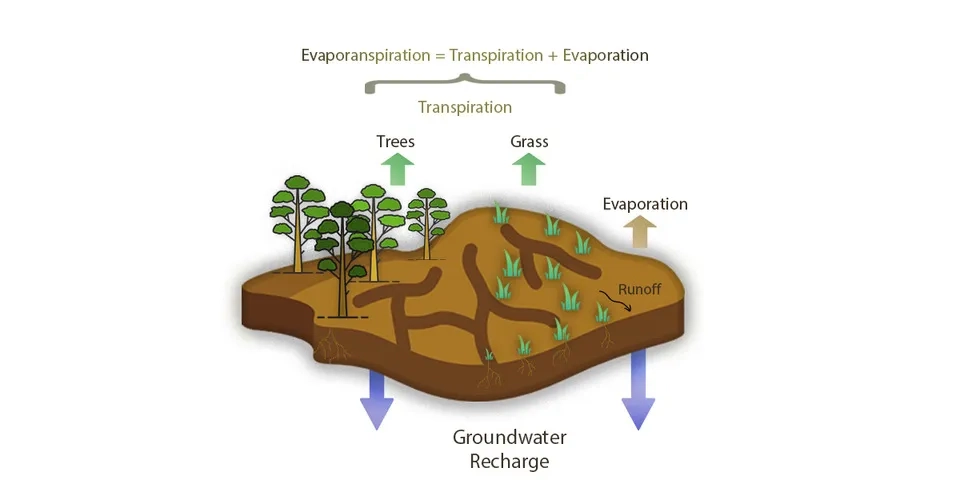
2.1.3. Evaporation (Evapotranspiration)
In shallow groundwater as well as in arid and semi-arid regions where evaporation exceeds precipitation due to weather conditions, evaporation plays a significant role in the salinization of groundwater. The process of dissolution and precipitation (especially in closed basins) turns soluble mineral compounds into solute chemical compositions that contain chloride, sulfate, and bicarbonate. Groundwater containing chloride is prone to increasing salinity through evaporation and salt dissolution. During the evaporation process, the water in the underground turns from liquid to gas and is transferred to the atmosphere, which causes a significant increase in the concentration of saline groundwater (Cary et al., 2015; Herrera et al., 2016).
2.1.4. Dissolution
In sedimentary basins, natural soluble salts accumulated underground are the source of groundwater salinity. Groundwater tends to become saline when passing through the subsurface and dissolving evaporate formations (halites) and carbonates. Also, by passing through ordinary aquifers and providing conditions for the dissolution of salts from the aquifer matrix, the underground water becomes brackish to salty. In addition, in mountainous areas, the downward penetration of rainfall and contact with rocks containing dissolved salt can lead to saline or brine, depending on the path of water and the concentration of dissolved salts. The effect of dissolution is greater in shallow aquifers and arid and semi-arid areas (Jia et al., 2017; Merchán et al., 2015).
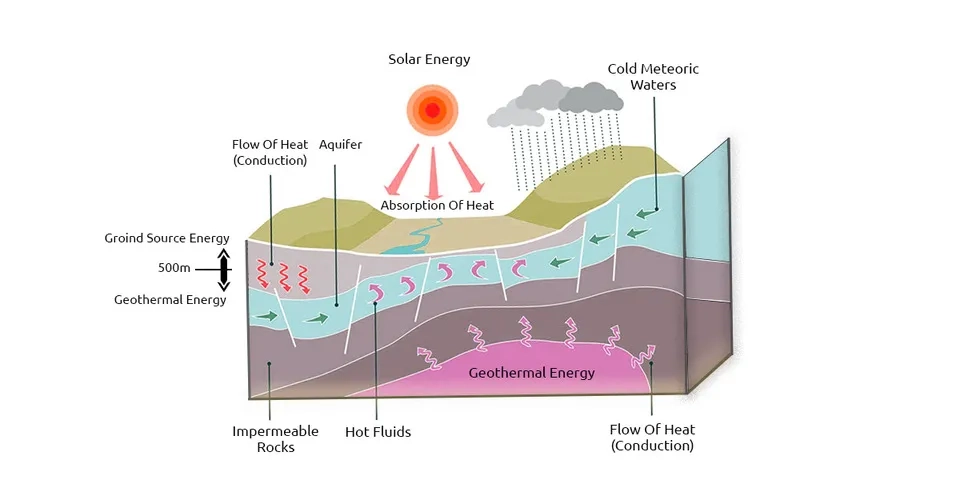
2.1.5. Geothermal Activities and Structures
Groundwater can move vertically and laterally in the aquifer system through channels caused by faults, fractures, and folds, depending on permeability, lithology, and physical characteristics. Channels resulting from geological structures provide a path for the infiltration of saline groundwater and affect the pattern of groundwater flow and salinization of freshwater aquifers. The high concentration of dissolved ions such as sulfate, sodium, calcium, and chloride in geothermal waters increases water-mineral reactions at high temperatures and pressures. Geothermal waters eventually reach the earth's surface and shallower depths, and the saline groundwater transfers to the earth's surface. Furthermore, saline fluids trapped in geological structures that are connected to active underground aquifers can lead to a gradual increase in salinity through advection and diffusion (Bundschuh and Maity, 2015; Sasaki et al., 2018).
2.1.6. Membrane Filtration
Membrane filtration, in turn, has a significant effect on the groundwater salinity in evaporite-poor sedimentary basins related to deep subsurface brines. Chemical compounds and compacted clays and shale in sedimentary basins cause the formation of a semi-permeable membrane that allows some molecules and ions to pass through. These geological membranes cause a delay in the passage of salty groundwater (especially containing calcium and chloride) and their accumulation on one side of the membrane (Tijani, 2004).
2.2. Anthropogenic Factors
Anthropogenic factors originate from human activities, which cause groundwater salinity on a smaller spatial scale compared to natural factors. The most important factors in groundwater salinization are discussed below:
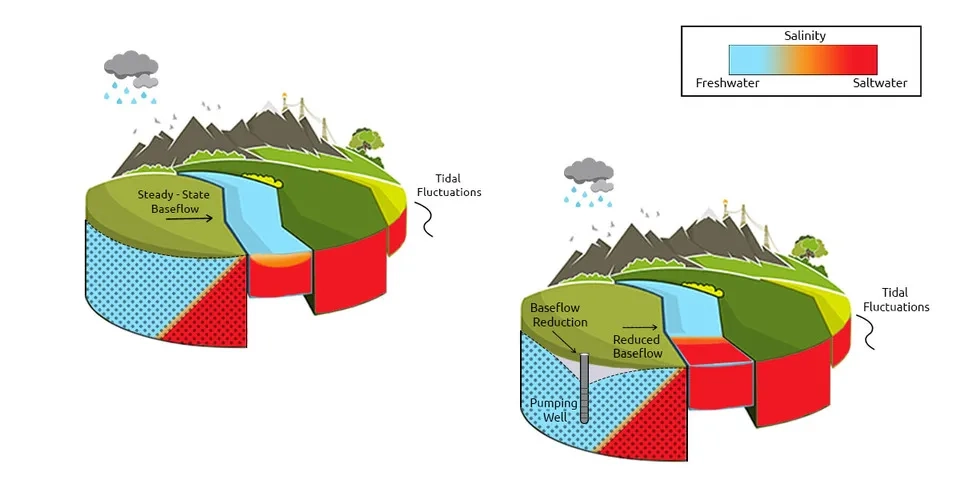
2.2.1. Groundwater Depletion
Excessive use of groundwater affects the quality and quantity of groundwater negatively. Among the quality characteristics of groundwater, salinity is contributing to groundwater depletion. The presence of brackish or saline groundwater at shallow depths and excessive pumping of groundwater lead to an increase in the contact surface between fresh and saline groundwater and groundwater salinization. Regions with high groundwater extraction and shallow saline groundwater, especially coastal aquifers, are very susceptible to groundwater salinization through groundwater depletion (Van Weert et al., 2009).
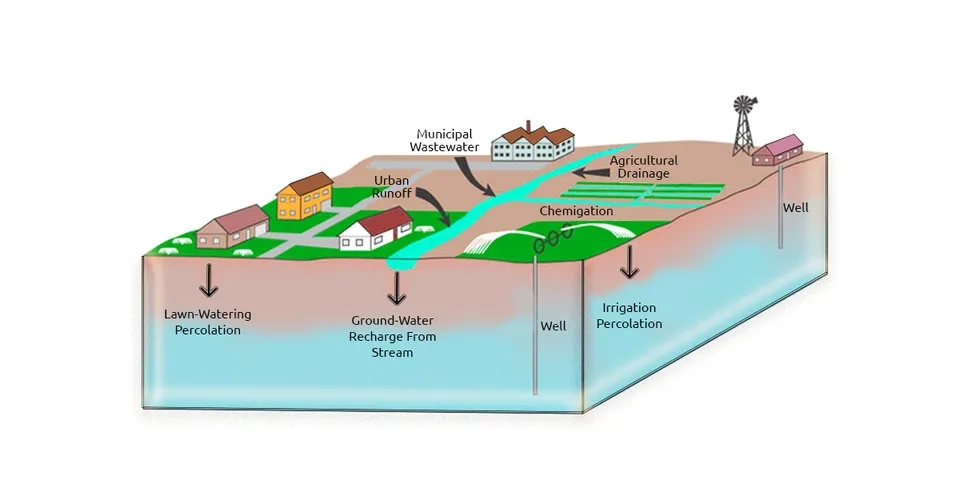
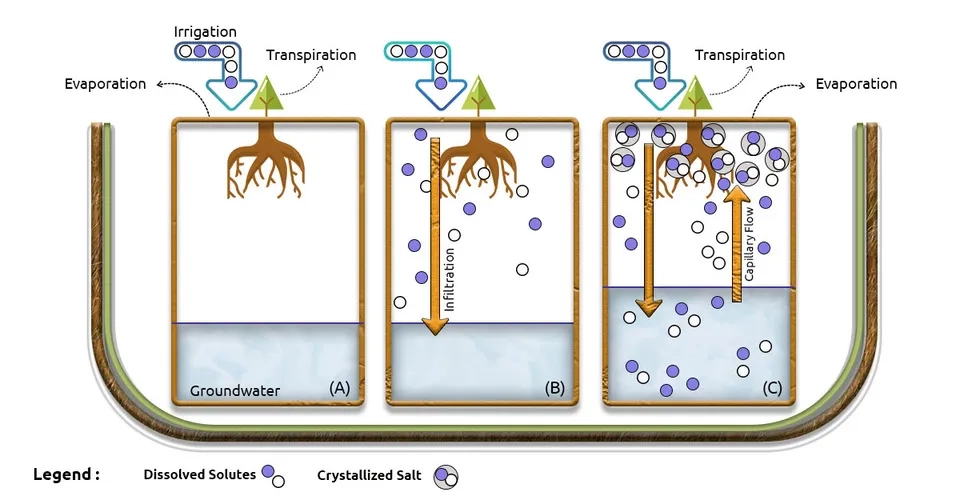
2.2.2. Irrigation
It is inevitable to use groundwater for irrigation all over the world, especially in arid and semi-arid areas. Irrigation increases groundwater recharge and groundwater table. On the other hand, evaporation transfers water vapor to the atmosphere and consequently, dissolved solids and salt remain in the root zone and near the surface. The remaining salt can penetrate deeper and reach the groundwater due to drainage and percolation. Furthermore, the salinity of surface water and wastewater and their use for irrigation can, in turn, increase soil and groundwater salinity (Bouzourra et al., 2014; Mora et al., 2022).
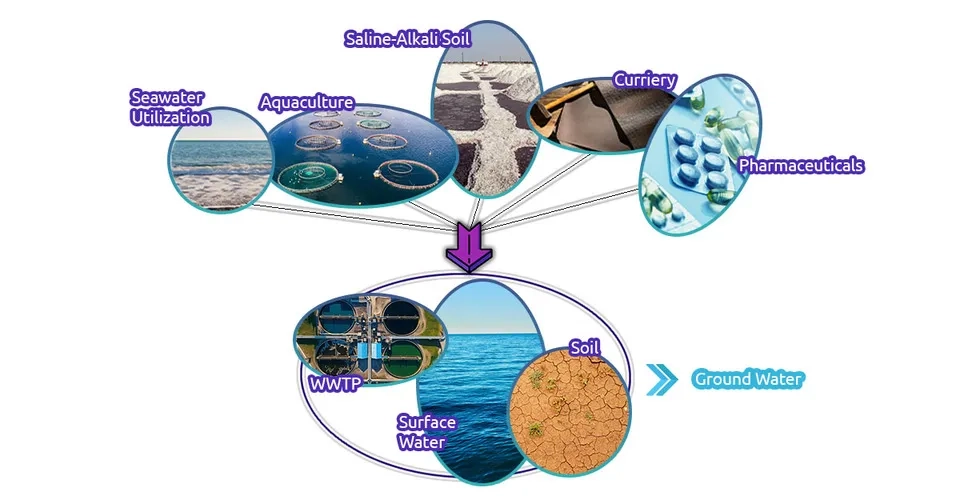
2.2.3. Wastewater
Wastewater treatment reduces the organic and inorganic compounds, ions, and nutrients in the wastewater and instead leaves soluble salts. Soluble salts and wastewater salinity originate from various industries (especially detergents) and agriculture. Disposing of saline wastewater in the soil and using this purified wastewater in irrigation transfers dissolved salts to the soil and groundwater, which ultimately leads to groundwater salinization (Russo et al., 2015)
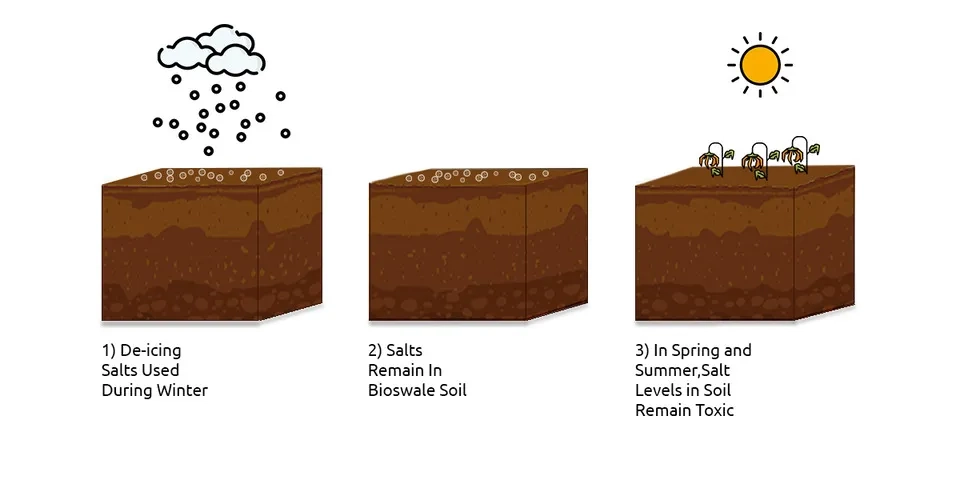
2.2.4. De-ice Salts
One of the uses of salt is to de-ice urban roads and highways and prevent freezing in cold seasons. The salts used for deicing are transferred to water sources in two ways: 1) leakage of salts stored in salt storage areas and 2) salt spilled on roads. By melting snow or dissolving salts in water, they are directly transferred to the groundwater, or they may first move to the surface water sources (lakes and rivers) and then penetrate into the groundwater. The majority of groundwater salinity is caused by deicing salt containing sodium chloride (NaCl) salt (Cooper et al., 2014).
3. Conclusion
Groundwater is a valuable source of fresh water that has faced challenges in terms of quantity and quality in recent years. Most of these challenges are caused by human intervention in the excessive use of groundwater and the introduction of pollutants through urban, agricultural, and industrial activities. One of the undeniable challenges related to groundwater quality is groundwater salinization so there have been reports of groundwater salinization and its unusability all over the world. The causes of salinity in groundwater are divided into two categories: anthropogenic and natural factors in groundwater salinization. Anthropogenic factors consist of groundwater depletion, irrigation, wastewater, and de-icing salts, while natural factors consist of seawater intrusion, connate saline groundwater, evaporation, dissolution, geothermal activity, and membrane filtration. Groundwater salinization covers a wide range of ecosystems and human life, and the effects on drinking water, food security, biodiversity, ecosystem, and infrastructure. Therefore, it is necessary to take preventive and sustainable policies to protect groundwater and prevent it from becoming saltier, to save plants, animals, and human lives in the future.
