
One of the most significant human concerns in the modern world is health care and the requirement for material disinfection. Has this issue affected your life? Do you know about hypochlorous acid and how effective it is for solving these problems? If you do not know about hypochlorous acid, join us as we explore this subject. Hypochlorous acid is widely used in water and wastewater treatment due to its strong disinfection capability. Hypochlorous acid is a non-toxic solution for disinfecting and it is completely safe for the human body. In this review, some functional advantages and disadvantages of electrolytic production of HOCl were identified. According to the commercial nature of this material, investigating detailed information is very challenging. In this review, data were collected from various sources. However, emails were sent to 110 companies that produce hypochlorous acid, but only four companies provided responses and cooperated. Therefore, this study aims to explore this subject in depth. The result of this review is the examination and classification of four models of the most important electrolytic cells for the production of hypochlorous acid, as well as the interesting applications of HOCl as a disinfectant solution in the water and wastewater treatment industry (Haddadzade, 2021).

In this era, one of the important human needs is access to health care and sanitization by safe disinfection solutions in order to live longer and protect their lives from harmful viruses and microbes. Most disinfection solutions are very toxic and hazardous, and using them on skin causes irritation and can also rust metals when in high-time contact with surfaces. However, all this danger does not exist with HOCl solutions. Most people have never heard of hypochlorous acid, but it is produced at a lower concentration in the immune system of the body by white blood cells. Moreover, hypochlorous acid is produced by water and sodium chloride salt using electricity in electrolytic cells, which is an efficient technology that has been studied. The behavior of hypochlorous acid in water directly influences its disinfection strength and stability across different pH ranges. In this review, hypochlorous acid and electrolytic production were investigated and especially the water industry application of hypochlorous acid was discussed (Balard A. J., 1834; Block, 2020).
1. Hypochlorous Acid (HOCl) Properties and Characteristics
HOCl is a disinfection solution that is produced in electrolytic cells with water and sodium chloride salt. It is a weak, unstable acid that naturally forms when chlorine dissolves in water and itself partially dissociates, forming hypochlorite. HOCl and OCl− are oxidizers. HOCl is the chemical formula of hypochlorous acid, and its molar mass is 52.46 g/mol. It has a simple molecule structure, and the center molecule is oxygen, which connects the chlorine and hydrogen atoms through a single bond. Furthermore, in the common representation, we can write its chemical structure as shown in the image below (National Center for Biotechnology Information, 2024):
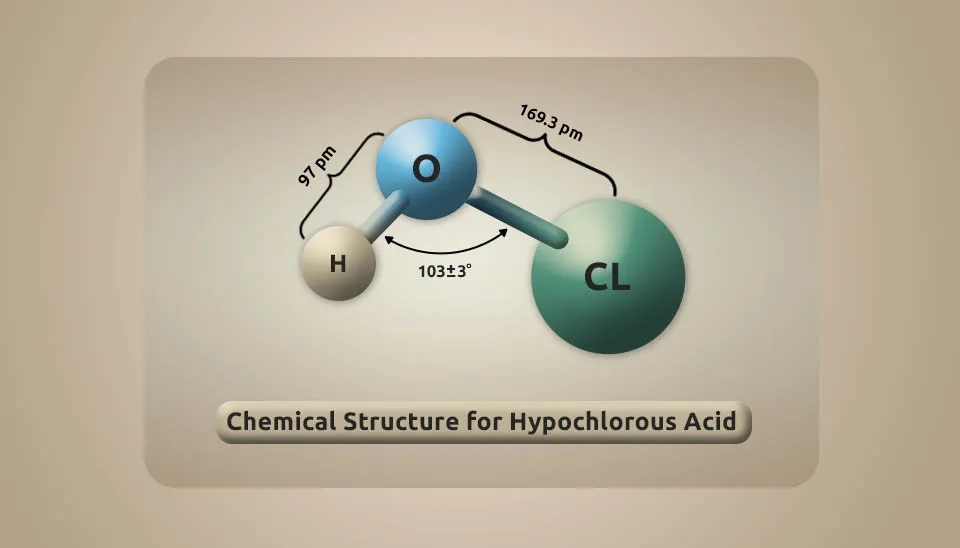
Hypochlorous acid only exists in aqueous solutions, which are colorless. Its chemical properties depend on its concentration. Hypochlorous acid is an oxidizing agent and has oxidation-reduction potential (ORP). ORP indicates that it can combat viruses. Millivolts [mV] is the unit of oxidation-reduction potential. The bacterial effect of HOCl depends on its ORP and FAC (free available chlorine) concentration. As we know, strong solutions for destroying the microorganisms and bacteria have a higher ORP. Moreover, FAC concentration and pH level are impacted by ORP. Therefore, a lower pH level and a higher FAC of HOCl will have a higher ORP. There are various ways to produce hypochlorous acid, such as adding chlorine to water, dissolving chlorine gas into the sodium hydroxide solution, dissolving dichlorine monoxide in water, etc. In this review, the generation of hypochlorous acid by electrolytic production using softened water and sodium chloride salt and, in some cases, a safe acid will be studied (Suslow, 2004; Fair et al., 1949).
2. Applications of Hypochlorous Acid in the Water Industry
Drinking pure and clean water is one of the most important human needs for survival, and hypochlorous acid has applications in the water and wastewater industry. Therefore, HOCl has the potential to solve the problem. Join us to deeply explore hypochlorous acid as a disinfection component in the water and wastewater.
2.1. Water Disinfection and Purification
Hypochlorous acid has a wide range of applications in various industries. As we mentioned above, one of these important applications is water disinfection and purification. First of all, water purification must be used to produce HOCl because hardness in water can cause damage to these cells and might increase the cost of maintenance and services. Therefore, implementing this process is a beneficial way to reduce the operational costs associated with producing and storing hypochlorous acid. Therefore, hypochlorous acid with an FAC concentration lower than 500 ppm is non-toxic and completely safe for humans and could be used for water disinfection. It could kill 99.9% of bacteria and pathogens. For many years, chlorine-based solutions have been used for disinfection of pool water and to prevent transmission of disease into human skin from pool water. Moreover, a chlorine-based solution is sprayed on water in one step of the water treatment process; that is called the chlorination step (Patent, 1990). Nowadays, hypochlorous acid serves as an excellent alternative to these compounds, offering a safe and highly effective solution for disinfection without causing harm.
Hypochlorous acid is a chlorine-based solution that is effective against viruses and diseases. It could be used in water treatment industrial plants as a chlorine-based solution, which is safe and non-irritating for human skin. Electrolyzed water is one of the common names of hypochlorous acid in industry; the concept of electrolyzed water was generally developed in Russia and used for water contamination and water regeneration that is provided in the next (Orejel. et al., 2020; Adal et al., 2024).
2.2. Wastewater Treatment and Reuse
In wastewater treatment, hypochlorous acid or electrolyzed water is used. The chlorination method is used for killing pathogens in wastewater treatment. Wastewater requires disinfection by hypochlorous acid because it is efficient and cheap. As is known, hypochlorous acid is produced by water, sodium chloride salt and electricity in electrolytic cells. These compounds have lower prices, and this method for purifying water is very economical and environmentally friendly. For producing hypochlorous acid from water and salt, a lower amount of electricity must be used, and two or three operators are required to operate the process of HOCl production, which means that the only pollution in this system comes from electricity generation. Therefore, this method is environmentally friendly due to the fact that it causes less damage to plants and minimizes global warming (Patent, 1990).

Hypochlorous acid is used for cleaning water distribution pipes and wastewater treatment. These pipeline systems are used for transporting water and wastewater, purifying water to homes, and sending wastewater to sewage plants for treatment. Viruses accumulate in these pipes during the distribution process. Moreover, hypochlorous acid can be used for cleaning these pipes, the same as the chlorination for water treatment and pool disinfection. Additionally, this disinfection method helps improve the health and sanitization of the environment (Taylor and Francis Group, 2018).
3. Electrolytic Production Methods of Hypochlorous Acid
There are many ways to produce the hypochlorous acid solution, such as dissolving dichlorine monoxide in water, the reverse reaction of sodium hypochlorite with hydrogen peroxide, and other methods that are mentioned above. Moreover, a new company has recently developed some tablets that could be dissolved in a specific volume of water, such as effervescent tablets and hypochlorous acid. All these methods of producing hypochlorous acid are medical ways and they produce hypochlorous acid with lower stability and shelf life compared to electrolytic technology. One of the engineering methods for producing HOCl is using electrolytic cells. In this section, all the electrolytic cells that produce hypochlorous acid will be reviewed (Wilberg et al., 2001).
3.1. Overview of Electrolytic Technology
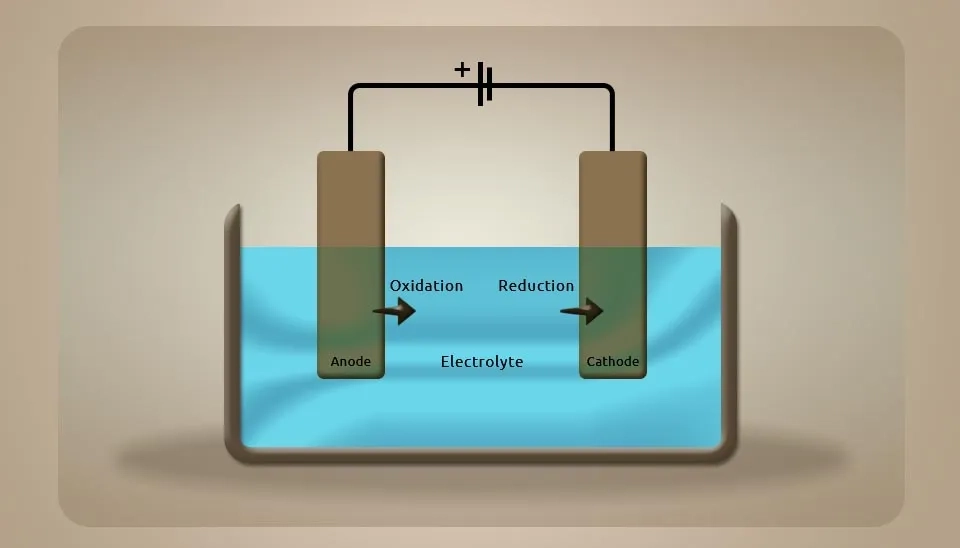
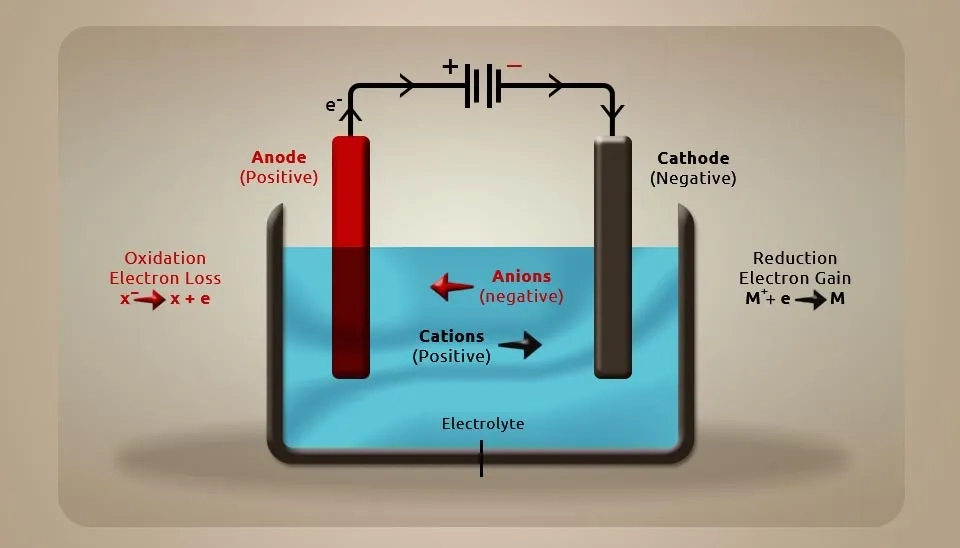
Electrolytic cells are one of the electrochemistry cells that work with an external circuit and interchange ions and atoms by adding or removing electrons. In these cells, the reduction and oxidation reactions occur ; the loss of electrons in the oxidation is called oxidation, and the gain of electrons is called reduction. Moreover, two types of electrodes must be used for the cathode and anode in these cells. In these technologies, there are four main components such as the cell body, power source (DC), Electrolyte, and electrodes. Changes in these main components create variables of the system, such as differences in voltage, current, time duration, composition of electrodes, and electrolyte properties. In some technologies, a divider between electrodes can change the mode of operation of these systems. Moreover, spacing between electrodes and electrode configuration can change the result of the experiment (Faraday, 1894; C. Perry et al., 2020; L. Balogh et al., 2021).
In this paper, the important factor of producing hypochlorous acid is the electrolysis process and according to Arthur’s classification and cells with or without a divider, four types of electrolytic cells for producing HOCl are selected that must be reviewed. In the following, some advantages and disadvantages will be identified. These electrolytic cells, which can operate in a continuous and batch process, have a flow rate of zero in the batch process and feed material is placed inside the cells. After a specific time, the products, hypochlorous acid , are produced. But in the continuous process, the flow of feed material is not zero and feed enters the cell at a specific flow rate, then the product leaves the cell with a specific flow rate. One of the important factors in these hypochlorous acid generators is the shape of the electrodes, which are cylindrical electrodes and plate or prismatic electrodes. The cylindrical shape is more stable than plate electrodes, which means that using cylindrical electrodes can increase the efficiency of the system (Bakhir et al., 2018).

3.2. Equipment and Operational Mechanisms
In this part, currently, there are four models of electrolytic cells to produce the hypochlorous acid, and these models have advantages and disadvantages that will be reviewed. Each of these models has a product of hypochlorous acid with a pH level because of the existence of this acid in a wide range of pH and FAC concentration. The feed for these cells is water and sodium chloride salt and in some special cases, water and hydrochloric acid (HCl) are used . The classification of these cells is according to the divider between electrodes, which will be studied (Haddadzadeh, 2021).
3.2.1. Simple Cells Technology
This model of electrolytic cells for producing hypochlorous acid is the simplest and oldest technology, which is used in the laboratory. First time, Jérôme Balard discovered the hypochlorous acid, which was used during WW1 and WW2 to clean and disinfect the wounds of the war-injured. This model is an operating batch, and there is no other model that operates continuously. This is not an industrial model of a hypochlorous acid generator but can be selected for experimental or domestic use. Feeding these cells is water and sodium chloride salt, and in some devices, vinegar is used for controlling pH levels. The pH level of hypochlorous acid from these cells must be controlled in the range of 4 to 7 (Balard A. J., 1834).
The mechanism for working these devices is basic, requiring specific softened water, NaCl salt, and vinegar or lemon juice. These materials are placed in the cells and after a specific time, hypochlorous acid is ready. The concentration of hypochlorous acid depends on the residence time of contacting the electrolyte—feed material—to the electrodes. Therefore if softened water and NaCl salt remain in simple cells, concentration will be increased (Patent, 2019).
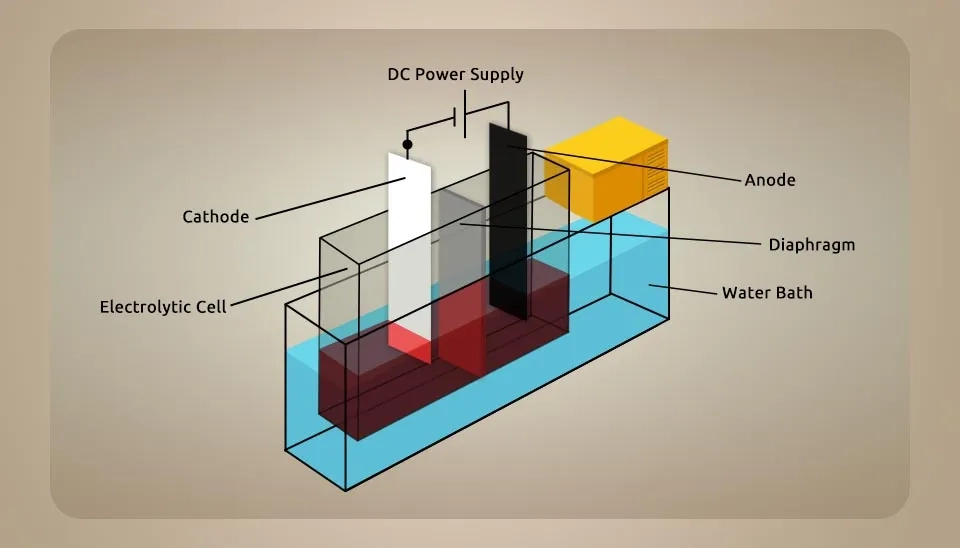
3.2.2. Divided Cells by Diaphragm Technology
This model of electrolytic cells that produce hypochlorous acid is one of the oldest technologies, which divides the electrodes and two types of electrolytes: anolyte and catholyte. In the catholyte zone, sodium ions (Na⁺) and water accumulate around the cathode electrode, and in the anolyte zone, chlorine molecules (Cl2) and water accumulate around the anode. In this case, anolyte always contains hypochlorous acid and catholyte mostly contains sodium hydroxide. These devices can operate in both batch and continuous modes, but the continuous operation is more common and widely used for industrial applications. These devices have two types of products: hypochlorous as a product and sodium hydroxide as a by-product. Feeding these cells is softened water and sodium chloride salt. These cells are required for installation because water must enter at higher pressure to facilitate the moving ions through the diaphragm. Moreover, filtered water must be used because water with hardness can decrease the efficiency of the divider and cathode electrode. These hardnesses, such as calcium and magnesium, can remain in divider pores or foul around the cathode, which means decreasing the efficiency. About diaphragm, It is a material with average pores less than one micron and has a larger pore size and lower efficiency than a membrane. It is preventive to mixing the catholyte and anolyte and could be made by different materials, such as woven fabrics, nylon, fiberglass, polyvinyl chloride (PVC), etc. Moreover, diaphragms have different types, such as ceramic diaphragms, piezoelectric diaphragms, ion-selective diaphragms, and porous diaphragms (Gluhchev et al., 2015; patent, 1999; patent, 1959; Patnaik et al., 2015).
3.2.3. Divided Cells by Membrane Technology
This model of electrolytic cells that produce hypochlorous acid is one of the newer technologies, which has a membrane divider between electrodes and two types of electrolytes: anolyte and catholyte. In the catholyte zone, sodium ions (Na⁺) and water accumulate around the cathode electrode, and in the anolyte zone, chlorine molecules (Cl2) and water accumulate around the anode. In this case, anolyte always contains hypochlorous acid, and catholyte mostly contains sodium hydroxide. These devices operate continuously and are used for industrial use. These devices have two types of products: hypochlorous as a product and sodium hydroxide as a by-product (Henry, 2013; patent, 2013).
Feeding these cells is softened water and sodium chloride salt. These cells require installation because water must enter at higher pressure to facilitate the moving ions through the membrane. Moreover, filtered water must be used because water with hardness can decrease the efficiency of the divider and cathode electrode. These hardnesses, such as calcium and magnesium, can remain in divider pores or foul around the cathode, which means decreasing the efficiency. These devices are very common for hospitals because both products from these cells will be used. Using sodium hydroxide for cleaning the surface and using the hypochlorous acid for sanitizing and disinfecting wounds. On-site generators of the membrane-divided cells are industrial and can be used in many hospitals in Russia, Japan and the Commonwealth of Independent States (CIS), as well as in many medical centers, hotels, public centers, etc.
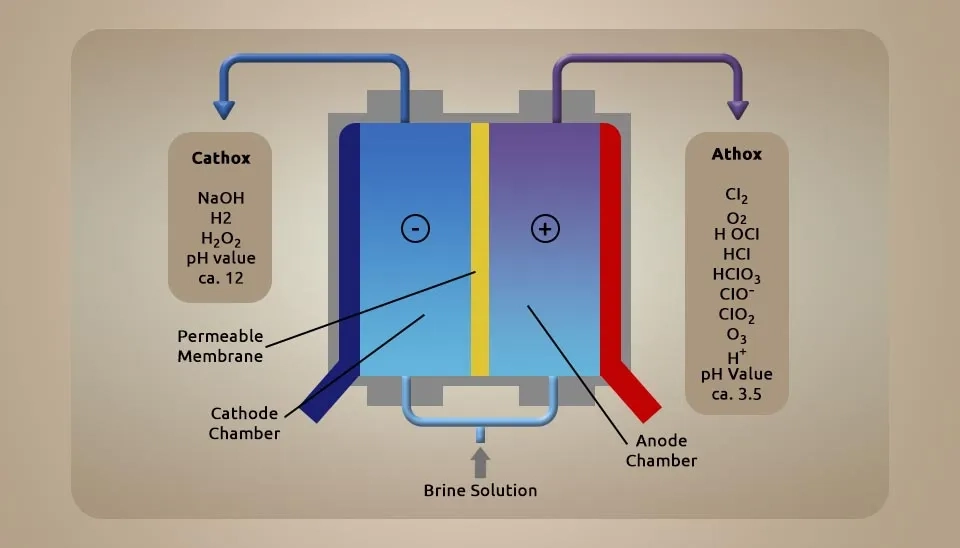
These cells produce hypochlorous acid and sodium hydroxide. Sodium hydroxide is an excellent antiseptic for surfaces such as ceramic, all the materials in hospital floors, etc. About membrane, It is a porous material exactly like a diaphragm but has a smaller pore size and higher efficiency than a diaphragm. It is important to prevent the mixing of the catholyte and anolyte, which can be made by various materials such as polymers, sulfonated nafion, polypropylene (PP), ceramic, metal materials, etc. Also, diaphragms have different types, such as electro-membrane, ion-exchange membranes, bipolar membranes, dialyzer membranes, ceramic membranes, horizontal membrane and dual membranes (Patent, 2012; patent, 2011; vasudevan S, 2013; Veasey, 2016)
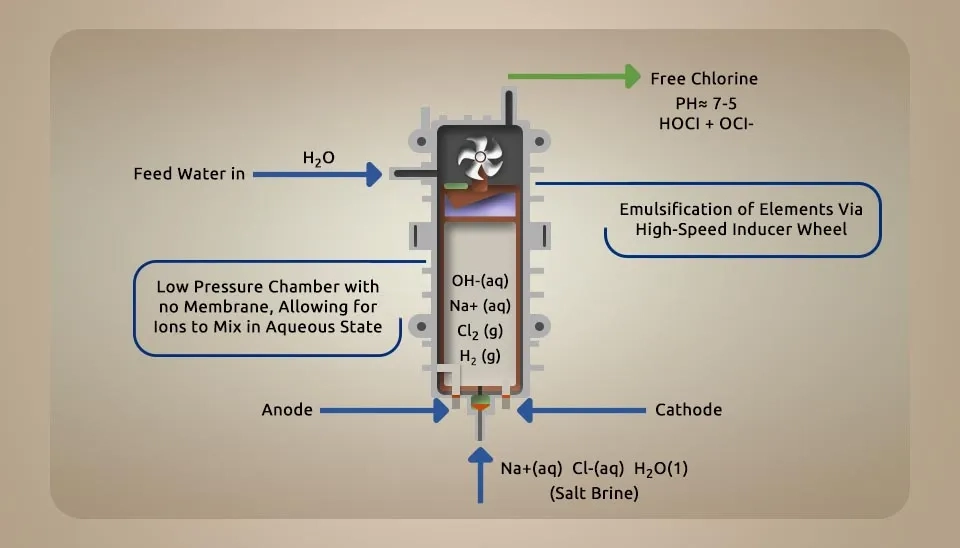
3.2.4. Single-Cell Technology
This model of electrolytic cells that produce hypochlorous acid is the latest and smartest technology, which has a pH controller. This technology controlled the pH level by emulsifying electrolyte elements via a high-speed inducer wheel for better and more efficient mixing. In this case, hypochlorous acid is continuously produced and used for industrial purposes. These devices have a simpler design that divides cells because they do not have dividers. Feeding these cells is water, sodium chloride, salt, and hydrochloric acid (HCl). These cells do not require installation because they lack dividers, making them cheaper and easier to maintain. Therefore, tap water must be used. According to Haddadzadeh (2021), “One of the main and common problems in this model is the bubble formation in the cell (hydrogen gas is produced as bubbles), which reduces the efficiency of the cell because it decreases the available surface area for electrolysis. The combination of high current and temperature causes the bubble generation in the electrolytic cell." (Naka et al., 2020; Jafry et al., 2017; Gessi et al., 2023)
3.3. Advantages and Disadvantages (Simple Cells, Divided Cells by Diaphragm, Divided Cells by Membrane and Single Cells)
All these technologies that are reviewed in the last parts have advantages and disadvantages, which are related to production costs, start-up costs, maintenance costs, operation methods, feed material quality, stability and shelf life of hypochlorous acid, etc. One of the important advantages of these types of generators is their low operation cost. In general, operating expenses include the required number of operators and the cost of feed materials. In this case, hypochlorous acid is produced from water and sodium chloride salt, which are cheap and available components to use. Furthermore, 2 or 3 operators are required for operating the process. Therefore, producing hypochlorous acid in electrolytic cells has low operation costs.
The important disadvantages of these generators are high start-up costs and limited hypochlorous acid shelf life. Electrolytic cells for producing hypochlorous acid are extremely expensive because of all the materials that are used in these structures. Usually titanium electrodes coated with mixed coatings of iridium dioxide, ruthenium dioxide, and platinum are used for anode electrodes because the oxidation reactions occur around the anode and it must have protection and for the cathode, pure titanium and pure platinum are used, which are very expensive materials. ABS material is used for cell bodies, and it has a reasonable cost. Moreover, PLC and, in some cells, a divider exists, which means higher cost for hypochlorous acid generators by electrolytic cells. Therefore, these devices are very expensive, which is a problem for startups. Hypochlorous acid produced by electrolytic cells has a longer shelf life but is limited and exposed after 6 to 12 months. Therefore, marketing and export markets are very critical and have difficulty. It is recommended that these technologies be placed in hospitals and health care centers, where they are produced and consumed immediately with hypochlorous acid on-site (Haddadzadeh, 2021).
In the table, all the advantages and disadvantages of these four technologies are as follows:
Table 1. Advantages and Disadvantages for Simple Cells, Divided Cells by Diaphragm, Divided Cells by Membrane, and Single Cells.
Type of HOCl Cells | Advantages | Disadvantages |
Simple Cells | Batch operation | Low production capacity |
Divided Cells by Diaphragm | Continuous operation | High energy consumption |
Divided Cells by Membrane | Continuous operation | High energy consumption |
Single Cells | Continuous operation | Bubble formation in high current and high temperature |
Download Full Table of Advantages and Disadvantages for Simple Cells, Divided Cells by Diaphragm, Divided Cells by Membrane, and Single Cells.
3.4. Energy Efficiency and Sustainability (Instruction for Feed Material and Cell Parts Maintenance)
In these types of cells for producing hypochlorous acid, as mentioned above, softened water or tap water, sodium chloride salt, and hydrochloric acid are feed materials. Water must be purified for divided cells because of the presence of dividers, such as diaphragms and membranes; the hardness remains in the pores, which decreases the efficiency of the system. In the remaining technology, tap water will be used. Moreover, sodium chloride must have 99% purity in divided technology because of the presence of the divider and cathode (Wilberg et al., 2001; Andrew K. Boal, 2009).
According to Haddadzadeh (2021), “The chemical and physical properties and composition of hypochlorous acid will vary depending on the concentration of brine solution, pH level, voltage, current density, temperature of electrolyte, time of electrolysis, mode of electrolysis operating, etc. Antimicrobial and sanitizing effects of hypochlorous acid depend on these parameters. The amount of current density has an effect on the efficiency of the electrolytic cells. Current density is an important factor in electrolysis.” The efficiency number for any real system is not one hundred, so maintenance must be determined. After a specific time, fouling formation occurs around the cathode electrode and in the divider cells; some hardness remains in the divider pores, and these issues decrease the efficiency of the system, which means that cleaning should be done. Washing of these cells must be done by a water jet system. A water jet (high pressure) is usually used with water and muriatic acid for cleaning (Filimonova, 2020). These technologies have recently employed monitoring and control systems to enhance the quality and stability of hypochlorous acid. These technologies typically use a programmable logic controller (PLC) to achieve this purpose. These controllers have three main parts: a software part and two hardware parts. The hardware part (temperature sensors, pressure sensors, pH sensors, conductivity sensors, etc.) is monitoring the chemical properties of the system’s product, which sends a signal to the software part. It is checked and converted into a control signal and sent to the last hardware part for controlling the parameter of the chemical properties of the product (Patent, 2013).
4. Environmental and Health Safety
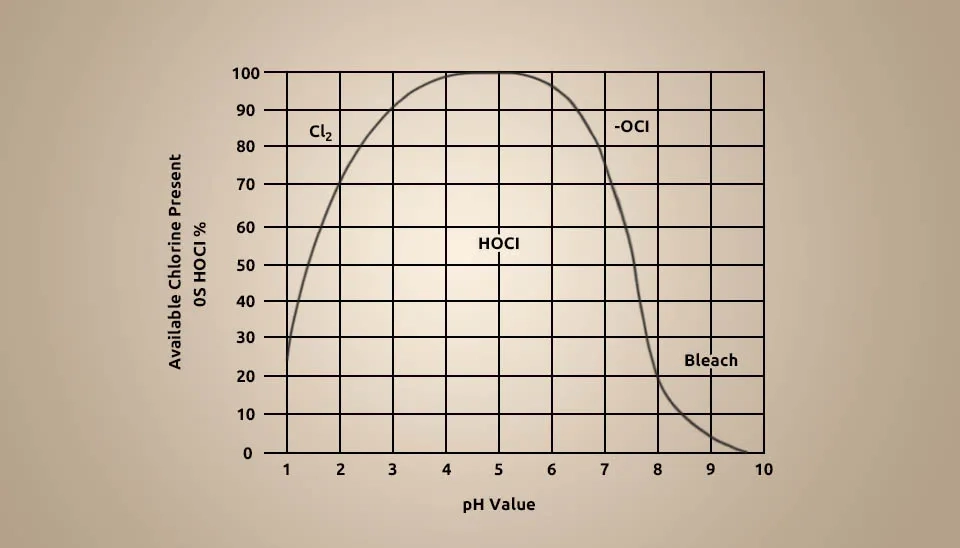
The health and safety of hypochlorous acid depend on some main parameters, such as pH level, FAC concentration, and stability, which are studied. PH level affects the forms of free chlorine in an equilibrium. According to Fig. 11, At a temperature of 25°C, if the pH level is below 1.5, the free chlorine gas exists in the aqueous solution; if the pH level is above 7.5, the hypochlorite ion (OCl⁻) and sodium hypochlorite (NaOCl) exist in the aqueous solution; and if the pH level is between 1.5 and 7.5, the hypochlorous acid exists in the aqueous solution, which means the pH level must be controlled in this range for health safety and more effectiveness (Y. Kott et al., 1974; Al-Haq et al., 2005).
The stability and shelf life of hypochlorous acid depend on pH level, ORP, and FAC concentration. When the pH level is controlled in this safety range, the HOCl has a higher shelf life. Moreover, if the pH level is controlled at 5, the shelf life of HOCl might exceed a few months. The quality of feed material has a significant impact on the stability of HOCl. When softened water, which is produced by reverse osmosis water treatment or other water purification technologies, is used, the stability of hypochlorous acid is increased (Block, 2020). There are some instructions for storing hypochlorous acid to increase the shelf life of it. HOCl has a partial dissociation in water and the dissociation constant (Ka) of it is 3×10⁻⁸ at 25°C. This number is relatively high and to prevent the dissociation of hypochlorous acid, the type of package storage must be specified. According to Block (2020), when the HOCl solution was exposed to sunlight, the chlorine reduction started on day four. When it was sheltered from sunlight, the chlorine reduction started after day 14. The half-life increases with decreasing pH owing to the decreasing ratio of OCl- to HOCl. The parts per million (ppm) is the concentration of the OCl-, which is the active ingredient and is known as the free available chlorine (FAC) in the solution. HOCl solutions are less stable when exposed to UV radiation, sunlight, or contact with air. The best material for packaging HOCl must block the sunlight, like opaque PET bottles, and also use a standard cap for this bottle to minimize the contact with air. HOCl should be stored in the refrigerator for a long shelf life (Rossi-Fedele et al., 2011; Issa-Zacharia, 2024).
5. Role in Disinfection and Sterilization
Hypochlorous acid has a wide range of applications in disinfection and sterility for different industries. In this part, some functional applications of HOCl will be reviewed. In the next section, water and wastewater applications of hypochlorous acid will be studied. Some applications of it are as follows (A. Mourad et al., 2019; Issa-Zacharia, 2024):
Water and wastewater disinfection
Agriculture sanitization
Meat sterilization
Livestock disinfection
Food sterilization
Hospital disinfection
The medical field disinfection
Home sterilization
Swimming pools disinfection
Mouth and teeth disinfection
Hand sanitization
Surface disinfection
Hint: Contact time between HOCl and the virus is an important factor, but this article did not mention the time required for cleaning with this required FAC concentration.

6. Conclusions
The present review aimed to investigate the applicability of hypochlorous acid, its production in electrolytic cells, and the various types of technology used for this purpose. Moreover, various details of these systems were studied, as well as the parameters affecting the quality and stability of HOCl, such as pH levels, voltage, current density, mode of operation, quality of sodium chloride salt, quality of water, temperature of electrolyte, material of electrodes, etc. Using different models of these technologies was affected by chemical characteristic parameters of HOCl, such as pH, shelf life, ORP, FAC concentration, and conductivity.
Hypochlorous acid is a non-toxic, which is considered an effective disinfectant solution. The effectiveness depends on the amount of FAC concentration. When the free available chlorine concentration is higher than 500 ppm, HOCl could cause irritation on human skin but lower than 500 ppm is totally safe for humans and has a wide range of disinfection applications in different industries. Moreover, HOCl is used in the water and wastewater industries as a chlorination solution for killing pathogens and microbes in water and wastewater. Overall, hypochlorous acid plays a vital role in modern water and wastewater treatment due to its efficiency, safety, and ease of on-site production.
All the optimal operating conditions for producing hypochlorous acid by electrolysis were studied. There are four types of these technologies for HOCl production:
Simple cell, which is the simplest technology for producing hypochlorous acid
Diaphragm-divided cell, which is one of the older technologies for producing hypochlorous acid and has a divider (diaphragm) between the electrodes,
Membrane-divided cell, which is one of the newer technologies for producing hypochlorous acid and has a membrane as a divider,
Single cells, which are the smartest and newest technology for producing hypochlorous acid.
For the feed of these technologies, softened water and sodium chloride salt must be used, and usually for controlling pH in a specific range, one must use some safe acid or in domestic use, one could add some vinegar or lemon juice to control pH. Therefore, pH must be controlled in a specific range for all types of electrolytic cells for producing hypochlorous acid.
Simple cells or single cells: the pH range of the product must be controlled in 4 to 7.
Diaphragm-divided cells or membrane-divided cells: The pH of the catholyte must be controlled at 11 to 13, and the pH of the anolyte (HOCl) must be 2 to 4.
About divided cells, the quality of water and NaCl salt is important as feed for generators with a divider because hardness, such as iron, calcium, magnesium, etc., could damage divider walls and of fouling on cathode electrode walls. If these hardnesses get stuck in divider pores, it could decrease the efficiency of the present technology, cause formation fouling on cathode electrodes, and increase the consumption of power. Moreover, this hardness can cause corrosion on anode electrode walls.

