
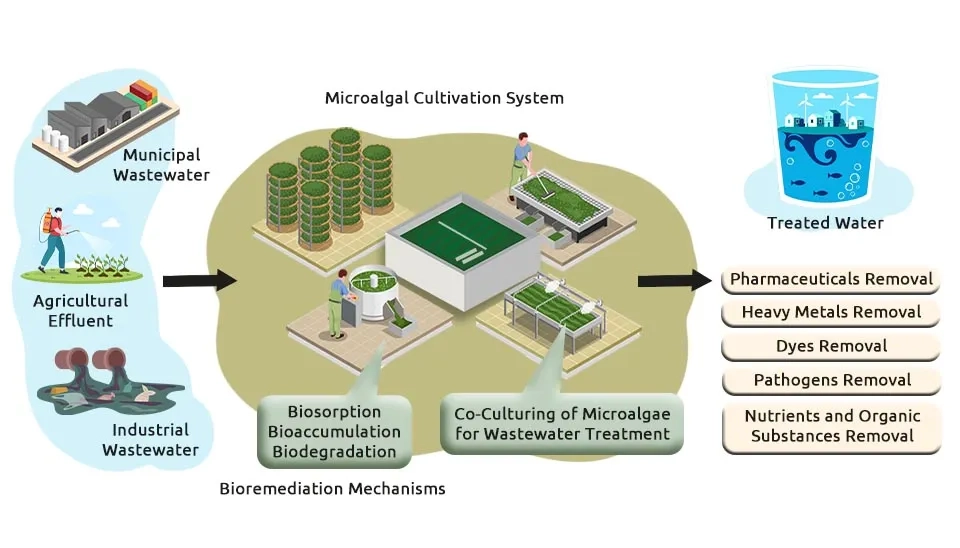
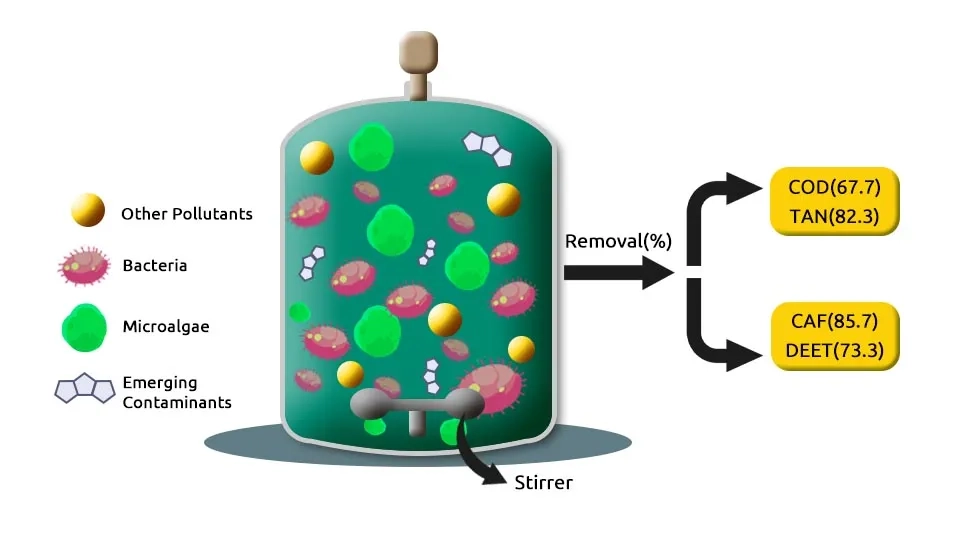
Dive into the world of cutting-edge environmental science as we explore a unique microalgae-bacteria consortium that promises to revolutionize the removal of multiple emerging contaminants in our water systems. This innovative consortium consists of a symbiotic relationship between specific microalgae strains and bacteria that work together to efficiently degrade and remove various emerging contaminants from water sources, such as 1) nutrients, 2) heavy metals, 3) antibiotics or pharmaceuticals, 4) Polycyclic Aromatic Hydrocarbons (PAHs), 5) pesticides, 6) herbicides, 7) manure, 8) insecticides, and 9) surfactants.
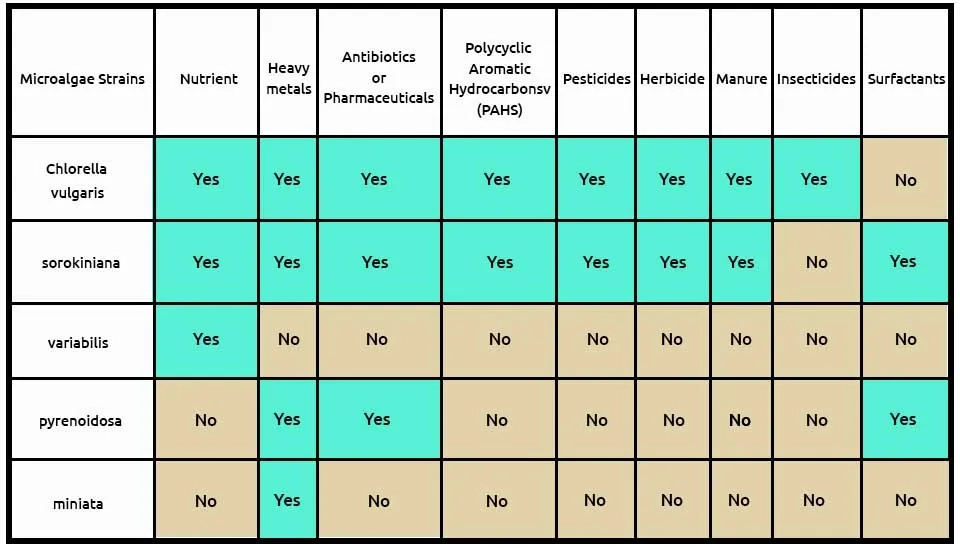
Microalgae play a crucial in the process by photosynthesizing and producing oxygen, which is essential for Microalgae Wastewater Treatment, as it creates an aerobic environment for the bacteria to thrive (Javed et al., 2022).
The Bacteria Consortia, in turn, break down the contaminants into harmless byproducts through a process known as biodegradation (Chan et al., 2022).
To Download MicroalGae-Bacteria Consortium To Remove Emerging Contaminants Click here
What sets the performance of the microalgae-bacteria consortium for pollution removal from wastewater apart is its ability to simultaneously target a wide range of contaminants, offering more comprehensive and sustainable solutions than traditional Biological Wastewater Treatment methods. Additionally, the consortium is highly adaptable and can be tailored to specific contaminant profiles in different water sources. Research and development in this field are ongoing, with promising results indicating the potential for widespread application of this technology in in treatment of Domestic Wastewater Sources, industrial facilities, and even household water filtration systems. This makes it an important addition to the range of Industrial Wastewater Treatment methods available today. By harnessing the power of natural processes, we can take significant strides toward cleaner and safer water for all (Kuhn et al., 2022). I'm thrilled to reveal that this groundbreaking work is detailed in our article, presenting compounds designed to purify nine crucial pollutants in wastewater. Follow our article to the end for a comprehensive understanding of the subject, acquaint yourself with these compounds, or even create your own formulations tailored to your local conditions.
-by-a-Microalgae-bacteria-consortium-in-wastewater-treatment-67948f4b9e42fb5ede56e551)
1. CC-A Microalgae-Bacterial Combination
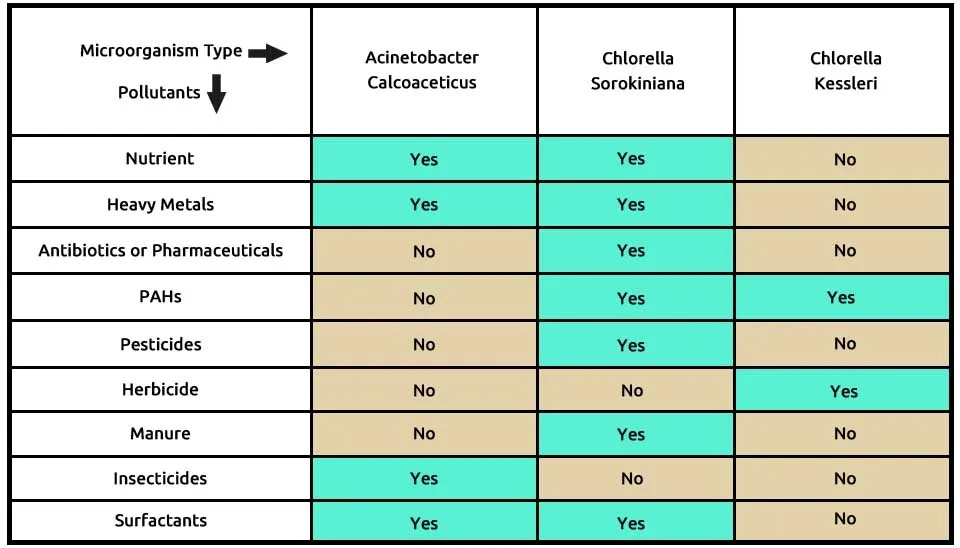
We suggest the first combination with the two microalgae, including C (Chlorella sorokiniana)and C (Chlorella kessleri), andone bacteria strain, A (Acinetobacter calcoaceticus), that can remove pollutants, according to Table 3. Chlorella sorokiniana removes many contaminants, such as nutrients from municipal wastewater, heavy metals, antibiotics or pharmaceuticals, PAHs, pesticides, manure, and surfactants. Chlorella kessleri withPAHs and herbicide removal can help. According to research, Acinetobacter calcoaceticus removes contaminants such as nutrients, heavy metals, pesticides, insecticides, and surfactants. The composition of microalgae, including Chlorella sorokiniana and Chlorella kessleri, andone bacteria strain, Acinetobacter calcoaceticus, in removing 9 pollutants from ECs is presented in Table 3.
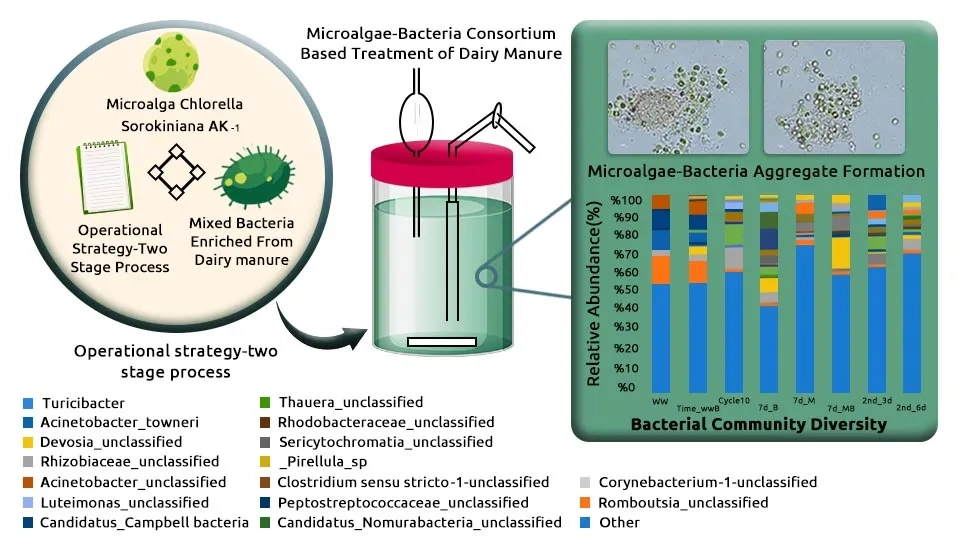
Chang et al. (2023) developed a functional microalgae-bacteria consortium (Chlorella sorokiniana, Chlorella vulgaris, Rhizobiaceae, Sericytochromatia_unclassified, Pirellula_sp, Owenweeksia, Devosia_unclassified, Acinetobacter_towneri, and Acinetobacter_unclassified) for the treatment of Dairy Manure Wastewater (DMW) using. They can remove COD, Biological Oxygen Demand (BOD), TN, NH3-N, and TP were 84.3%, 97.8%, 90.2%, 99.1%, and 100%, respectively, after 3 days. The conditions of strain growth were as follows: 20% unsterilized DMW, 1 L indoor photobioreactor, initial inoculum size of 0.4 g/L, 25°C, 150 rpm agitation, BG-11 medium, 0.2 vvm aeration with 2% CO2 for 5–7 days with continuous illumination at 300 μmol m−2 s−1. Makut et al. (2020) obtained a promising approach for producing crude oil via hydrothermal liquefaction of co-cultivation of microalgae and bacteria grown in large-scale open raceway pond utilization of paper industry wastewater as a cheap source of nutrients and water. They achieved 90% COD removal efficiency using a microalgae-bacterial consortium consisting of two microalgae, Chlorella sorokiniana strain and Chlorella sp., and two bacteria, Klebsiella pneumoniae strain and Acinetobacter calcoaceticus strain, after 25 days. Microalgae-bacteria inoculum ratio 1:1, initial pH of the medium 8.6, and microalgae-bacteria inoculum size 18% (v/v) under fluctuating sunlight intensity from zero (in the night) to a maximum of 2237 μE m−2 s−1 and temperature range of 23 to 39°C.
Mun˜oz et al. (2006) tested the residual algal-bacterial biomass for its ability to accumulate Cu(II), Ni(II), Cd(II), and Zn(II) with 50–100% removal efficiency after 35 days. Chlorella sorokiniana was cultivated in a Mineral Salt Medium (MSM) and a mixture of CO2/N2 (30/70% v/v) as a gas headspace. Ralstonia basilensis was cultivated in flasks supplied with 500 ml of sterile MSM containing sodium salicylate at 2 g L-1 and 1% (v/v) in a rotary shaker at 150 rpm under continuous illumination at 100 μE m−2 s−1 at 26°C for one week. Aparecido et al. (2021) evaluated the removal of Cephalexin (CEP) and Erythromycin (ERY) compounds often targeted by Pharmaceutical Wastewater Treatment Methods from a local Wastewater Treatment Plant (WWTP) effluent, mediated by microalgae-bacteria consortium bioremediation of emerging contaminants. They showed that the added concentrations of selected antibiotics did not restrain the consortium's growth. CEP and ERY were 96.54% and 92.38%, respectively, removed after the cultivation period. The inoculum containing the performance of the microalgae-bacteria consortium for pollution removal from wastewater was sampled from a natural eutrophic environment by Chlorella sorokiniana, and Brevundimonas basaltis was incubated at 120 rpm and 22°C under white Light-Emitting Diode (LED) with light/ dark cycles of 16/8 h of 235 ± 22 μmol photon m−2 s−1 for 7 days.
Goswami et al. (2019) discovered that a sustainable method of microbial biomass generated through co-cultivation of microalgae and bacteria along with wastewater remediation from the paper industry in a photobioreactor working in batch mode after 7 days was able to remove 99.95% of total nitrogen and 95.16% of COD. Chlorella sorokiniana, Chlorella sp. strain, and the bacteria Klebsiella pneumoniae and Acinetobacter calcoaceticus strain were cultivated with 18% (v/v) in an orbital shaker at 150 rpm, 30°C under 100 μE m−2 s−1 light intensity with a photoperiod of 16:8 h light and dark cycle, pH 8.6 for 7 days. Rasouli et al. (2018) used microbial biomass as a means to recover nutrients from industrial wastewater and upcycle them to feed-grade single-cell protein. They showed that an algal-bacterial consortium can remove 91% of the organic carbon present in the wastewater. Chlorella sorokiniana and Methylococcus capsulatus were cultivated in batch experiments 600 ml at 37°C under 2700 μE m−2 s−1 light intensity. Hamouda et al. (2020) investigated the microalgae/cyanobacterium consortium of Anabaena oryzae and Chlorella kessleri for bioremediation (100%) oil pollution after 14 days. microalgae/cyanobacterium consortium cultivated at room temperature (25 ± 1°C) on constant shaking at 50 rpm under natural daylight. According to the research conducted on these species, they are completely compatible and can remove pollutants at the same time.
2. CC-PP Microalgae-Bacterial Combination
According to Table 4, our second suggested combination consisted of the two microalgae, including C (Chlorella minutissima)and C (Chlorella vulgaris), and two bacteria, including P (Pseudomonas aeruginosa)and P (Pseudomonas stutzeri), that completely remove these 9 contaminants. Chlorella vulgaris is able to remove many pollutions, such as nutrients, heavy metals, antibiotics or pharmaceuticals, PAHs, pesticides, manure, and insecticides. With the election, Chlorella minutissima microalgaeforheavy metals, PAHs, and manure removal can obtain a synergy effect. PAHs, pesticides, insecticides, and surfactants are removed by Pseudomonas aeruginosa, while herbicides, insecticides, and surfactants can be removed by Pseudomonas stutzeri. The composition of microalgae, including Chlorella minutissima and Chlorella vulgaris, and two bacteria, including Pseudomonas aeruginosa and Pseudomonas stutzeri, in the removal of 9 pollutants from ECs, is presented in Table 4.
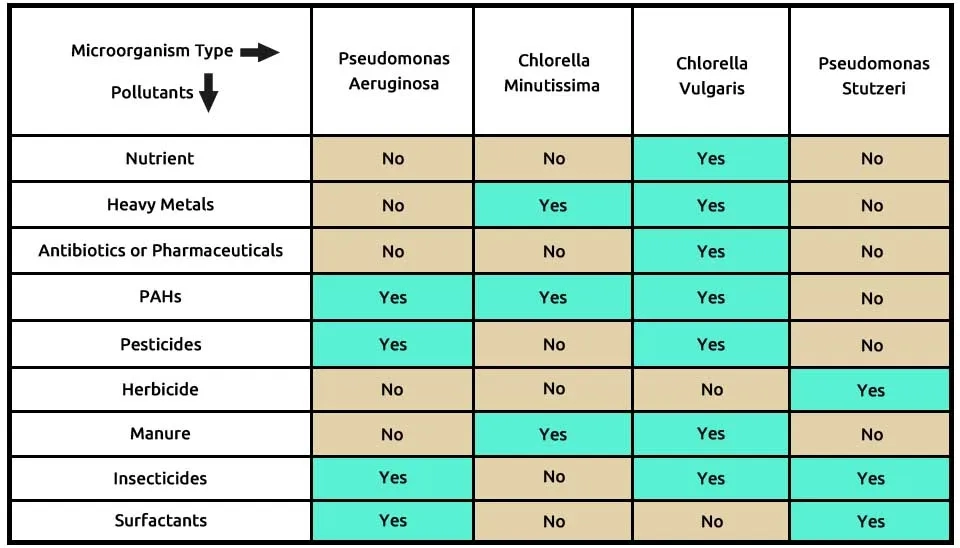
Khan et al. (2019) used Chlorella minutissima, Scendesmus spp, and Nostoc muscorum and their consortium for the biorefinery approach. Removal of NH4+-N, NO3--N, PO4-3-P, TDS, BOD5, and COD was found to be 92%, 87%, 85%, 96%, 90%, and 81%, respectively after 25 days. All strains were cultivated in Bold's Basal Medium (BBM) under solar radiation 30.78 and 20.64 MJ m-2d-1 at the growth period. Huo et al. (2020) investigated the co-culture of Chlorella and wastewater-borne bacteria (Bacillus firmus and Beijerinckia fluminensis) in vinegar production wastewater. They enhanced nutrient removal and influenced algal biomass generation. the highest COD, TN, and TP removal rates were improved by 22.1%, 20.0%, and 18.1%, respectively, at the end of the cultivation. The culture was placed at 25°C under a light intensity of 100 μmol m-2 s-1 in BG-11 medium mixed with increasing (10% to 100%) percentages of vinegar production with continuous shaking at 150 rpm under 5 days of cultivation. Safonova et al. (2004) studied the efficiency of algal-bacterial associations for the remediation of industrial wastewater using the algal strains Chlorella sp., Scenedesmus obliquus, several Stichococcus strains, and Phormidium sp. and the bacterial strains Rhodococcus sp., Kibdelosporangium aridum. Phenols were removed up to 85 %, anionic Surface Active Substances (anionic SAS) up to 73%, oil spills up to 96%, copper up to 62%, nickel up to 62 %, zinc up to 90%, manganese up to 70%, and iron up to 64 % and the reduction of the BOD25 and the COD amounted to 97% and 51%, respectively after 10 days. The bacterial strains were grown on an organic medium Nutrient Agar Siccum (NAS), pH=7.2 ± 7.4, incubated at 28°C illumination by 25 μmol photons/m2s supplied by 40Wcool-white fluorescent tubes after 7 days.
Castellanos-Estupiñan et al. (2022) removed nutrients and pesticides from agricultural runoff using microalgae and cyanobacteria consortium consisting of Chlorella and Scenedesmus sp. and Hapalosyphon sp. The highest removal efficiency of the pesticide (Chlorpyrifos) was obtained 100% with a biomass concentration comparable to a synthetic medium after 30 days. The media was mixed through the injection of filtered air with 0.5% (v/v) CO2 at a flow rate of 0.78 L min−1, 25°C, and light: dark cycle of 12:12 h at 100 µmol m−2 s−1. Lu et al. (2022) combined a novel wastewater treatment via a microalgae-bacteria consortium process with a sequencing batch reactor. They proposed a constructed wetland and microalgal membrane photobioreactor (Chlorella vulgaris and Iris pseudacorus) for toxicity reduction of Polycyclic Aromatic Hydrocarbons (PAHs). PAH removal (90.58%–97.50%) was achieved after 18 days. Aeration to maintain Dissolved Oxygen (DO) concentration, Mixed Liquor Suspended Solids (MLSS), and pH were at 0.5–1.0 mg/L, 2500–3000 mg/L, 7.0–8.0, respectively. Wang et al. (2020) investigated a microalgae-bacteria consortium, including Exiguobacterium and Bacillus licheniformis, Chlorella vulgaris, for piggery wastewater treatment. The final removal rates of Total Nitrogen (TN), TP, NH4+-N, and COD were 78.3%, 87.2%, 84.4%, and 86.3%, respectively, after 12 days. The bacteria were cultured in a heterotrophic medium, and the pH was adjusted to 7.0. The reagents used in the experiment were analytically pure. Microalgae was incubated on a BG-11 medium with an oscillation rate of 140 rpm, a temperature of 24.0 ± 1.0°C, the illumination was 50.0 μmol photons m−2 s−1 for 24 h, and the cultivation lasted for 7–8 days.
-removal-in-a-photo-electrochemical-system-with-a-photosynthetic-biofilm-679495c89e42fb5ede56e6a8)
Batool et al. (2018) employed microalgal (Chlorella vulgaris) -bacterial (Exiguobacterium profundum) co-cultures for the treatment of artificially prepared metal-rich wastewater. The results depicted that maximally, about 78.7%, 56.4%, and 80% of Cu, Cr, and Ni were removed, respectively, after an incubation period of 15 days. Microorganisms were cultivated in BG-11 medium, and the temperature and pH of the wastewater at the time of sampling were 32.4°C and 7.3, respectively. Zhang et al. (2022) completely removed Metronidazole (MNZ) from wastewater using electrochemistry-activated binary-species photosynthetic biofilm of Rhodopseudomonas Palustris and Chlorella vulgaris after 14 h. Rhodopseudomonas Palustris and Chlorella vulgaris are cultivated at a temperature of 27 ± 2°C and cold white fluorescent lamp (3000 Lux). Leong et al. (2023) investigated the feasibility of sustainable biohydrogen generation from dairy manure wastewater using a microalgae (Chlorella vulgaris) platform to achieve simultaneous wastewater treatment via a microalgae-bacteria consortium and clean energy production by Clostridium butyricum. The photobioreactor was run at room temperature, 200 rpm agitation, with 0.2 vvm 2% (v/v) CO2 aeration under continuous illumination by an external light source with a light intensity of 140 μmol m−2 s−1 with BG-11 culture media.
-treatment-and-biodiesel-production-6794967c9e42fb5ede56e6e3)
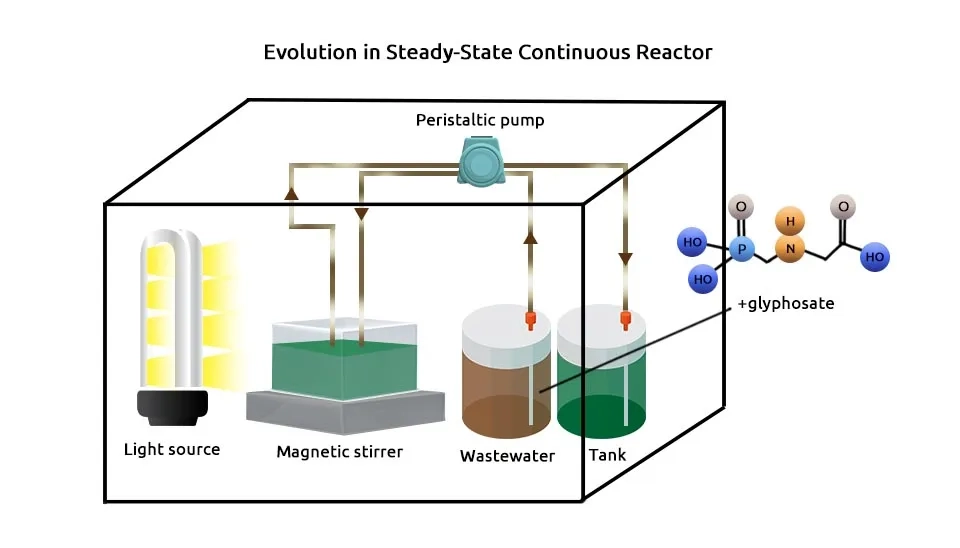
Sun et al. (2019) aimed to assess the performance of an Algal-Bacterial biocathode Photo-Bio electrochemical Fuel Cell (ABPBFC) operated with a daily light/dark cycle for simultaneous bioelectrical power generation and high-strength nitrogen removal (120 h) under Oxytetracycline (OTC) stress by adding different concentrations of OTC into the biocathode. Nitrogen removal (40–80%) and OTC degradation were stimulated at OTC concentrations less than 20 mg/L but inhibited at 50 mg/L. Consortium cultivated under alternating 9 h light/15 h dark cycles, pH=7, light source for cathode illumination 2500 lux, and constant temperature room (30 ± 1°C). Xie et al. (2020) investigated microalgal-bacterial (Chlorella vulgaris, Pseudomonas, Brevundimonas, and Hydrogenophaga) consortium in a Photobioreactor (PBR) for the Anaerobically Digested Center (ADC) treatment, the impact and degradation of micropollutant Sulfamethoxazole (SMX). Maximum SMX, COD, TN, and TP removal obtained 99.0 ± 0.2%, 72.12 ± 1.34%, 98.47 ± 0.69%, and 98.49 ± 0.73%, respectively after 7 days. Microalgae cultivated in the BG-11 medium. Borella et al. (2023) designed a bacterial consortium (Pseudomonas stutzeri, Comamonas odontotermitis, and Sinomonas atrocyanea) to remove glyphosate from polluted water, supported by the oxygen produced by a microalgal Chlorella protothecoides. Glyphosate concentration in both synthetic and real wastewater was reduced by about 53% and 79%, respectively. Bacteria strains were maintained in a 250 mL flask at ambient temperature in their suitable nutrient media. Microalgae were maintained in a 250 mL flask in a thermostatic chamber at 30°C in BG-11 medium continuously illuminated at 100 µmol m-2 s-1.
Omojevwe and Ezekiel (2016) biodegraded PAHs (anthracene, acenaphthene, dibenzo(a,h)anthracene, and benzo) using microalgal-bacterial consortia (92.09%) during 10 days. The consortia comprised Chlorella minutissimma and Aphanocapsa sp. as microalgae inoculants, while Citrobacter sp., Pseudomonas aeruginosa, and Bacillus subtilis as bacterial inoculants. Strains growth in BG-11 medium at 28 ± 2°C under sterile conditions. Li et al. (2020) enhanced the degradation of pyrene using two strains, Pseudomonas aeruginosa and Achromobacter sp. AC15 as a microbiological consortium. They show in the co-metabolism system of 600 mg/L pyrene and 1.4 g/L sodium citrate, and pyrene degradation reached 74.6%, 1.57 times, 2.06 times, and 3.89 times that of the mix-culture strains, single Pseudomonas aeruginosa and single Achromobacter sp. without sodium citrate, respectively after 14 days. Microbiological consortium cultures of bacteria were incubated with a Luria Bertani (LB) medium in a 250 mL orbital shaker set at 30°C and 200 rpm.
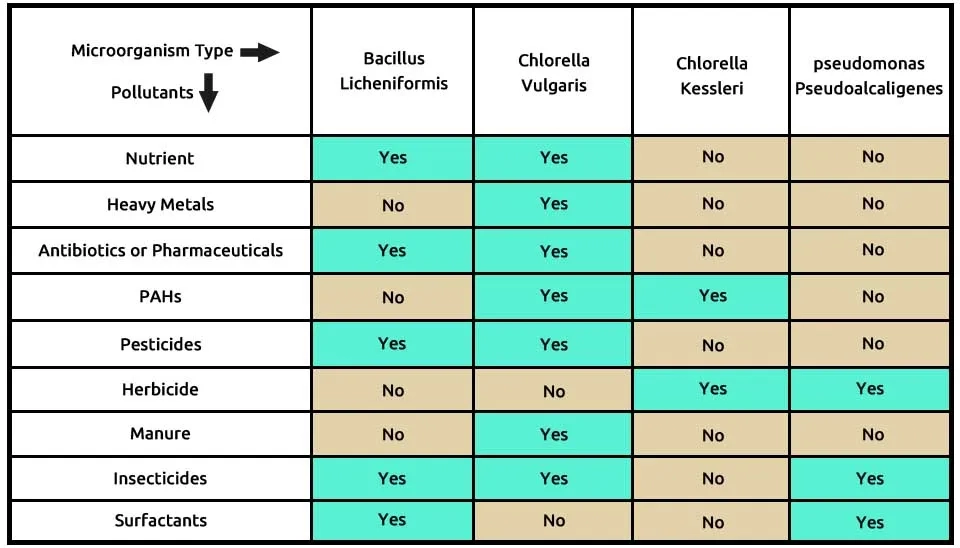
According to research, microalgae-bacteria consortium bioremediation of emerging contaminants, including Chlorella minutissima, Chlorella vulgaris, Pseudomonas stutzeri, and Pseudomonas aeruginosa, can be cultivated. These organisms can form a symbiotic relationship where the bacteria provide nutrients and growth factors to the microalgae while the microalgae produce oxygen and organic carbon compounds that can be used by these bacteria. However, it is important to carefully control the conditions of the culture medium, such as nutrient concentrations, pH, temperature, and light intensity, to ensure optimal growth of all organisms in the consortium. Additionally, monitoring the interactions between the microalgae and bacteria is crucial to maintaining a stable and productive consortium.
3. CC-BP Microalgae-Bacterial Combination
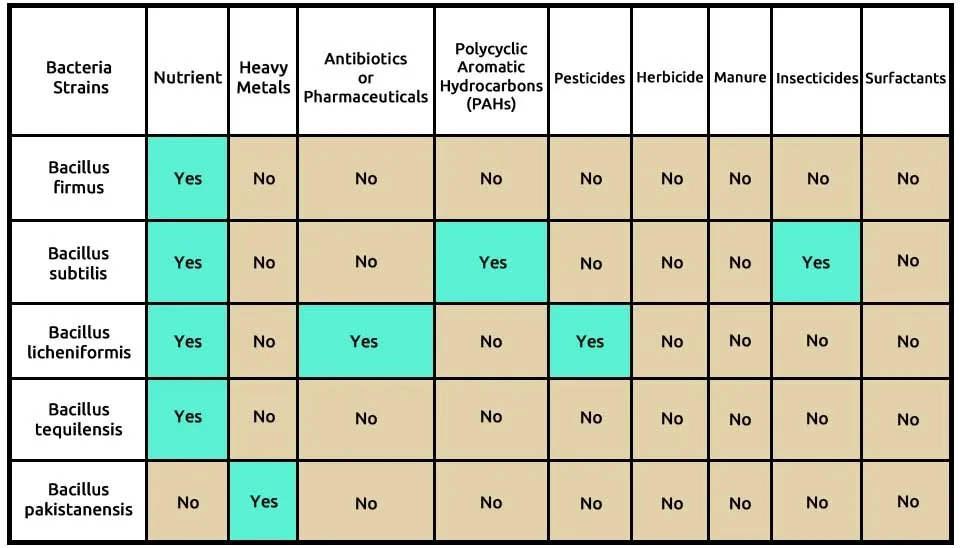
The third suggested combination used the 2 microalgae, including C (Chlorella vulgaris) and C (Chlorella kessleri), and 2 bacteria strains, including B (Bacillus licheniformis) and P (Pseudomonas pseudoalcaligenes), that completely remove these 9 contaminants, according to Table 5. Chlorella vulgaris is able to remove many pollutions, such as nutrients, heavy metals, antibiotics or pharmaceuticals, PAHs, pesticides, manure, and insecticides. Chlorella kessleri removes 2 pollutions such as PAHs and herbicides. Nutrients of wastewater, antibiotics or pharmaceuticals, pesticides, insecticides, and surfactants are removed by Bacillus licheniformis, while other bacteria, Pseudomonas pseudoalcaligenes, can help remove herbicides, insecticides and surfactants of the 9 contaminants. The composition of microalgae, including Chlorella vulgaris and Chlorella kessleri, two bacteria, including Bacillus licheniformis and Pseudomonas pseudoalcaligenes, in the removal of 9 pollutants of ECs, is presented in Table 5.
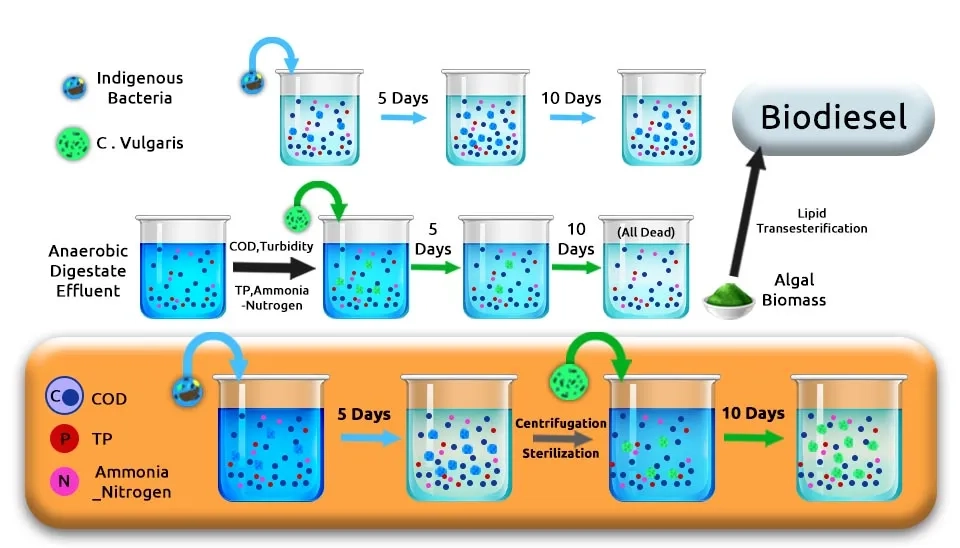
Gu et al. (2021) pretreated indigenous bacteria, which were selected from Pig Manure Anaerobic Digestate Effluent (PMADE) to alleviate their inhibition for the growth of Chlorella vulgaris after 5 days. Bacteria could decrease 34.04% and 47.80% of total phosphorus (TP) and turbidity in PMADE-1 and 80.81%, 43.27%, and 57.51% of COD, TP, and turbidity in PMADE-2, respectively. Microalgae were cultivated in BG-11 medium with a constant temperature of 26 ± 1°C and continuous illumination of 2.5 ± 0.1 kLux. The two wastewaters were stored in a freezer at 4°C and then autoclaved at 121°C for 30 min until further use. Mubashar et al. (2021) evaluated the performance of microalgae Chlorella vulgaris in an Enterobacter sp. assisted textile industry wastewater treatment system for decolorization (70%), removal of heavy metals such as Cu, Cr, Pb, and Cd (70–120% decrease), and COD (74%) after 5 days. Microalgae was cultured in BG-11 media at a light intensity of 60–80 µmol m−2 s−1 along with 2% CO2 mixed with an air and temperature of 25–28 ± 2°C followed by culturing in 5 L laboratory-grade plastic tubes for 7 days.
Guo and Tong (2013) cultivated a microalgae-bacteria consortium from Chlorella vulgaris and Pseudomonas alcaligenes, Elizabethkingia miricola, and Methylobacterium radiotolerans. They found Pseudomonas alcaligenes had a growth-promoting effect on C. vulgaris. In photoautotrophic conditions, enhanced C. vulgaris growth and increased extracellular organic carbon dissolved in the medium production and the chlorophyll content. C. vulgaris cultivated in the 3 N-BBM+V (BBM with 3-fold nitrogen and vitamins), pH 6.55–6.6, under room temperature and 14/10 h light/ dark cycle with 22.95 μmol photons m−2 s−1 was for 8 days. Bacteria were incubated at 37°C in a dark incubator on similar agar plates at 250 rpm for 48 h. Wang et al. (2020) screened a Chlorella-bacteria consortium consistent with Exiguobacterium and Bacillus licheniformis for piggery wastewater treatment. Removal rates of TN, TP, NH4+-N, and COD were 78.3%, 87.2%, 84.4%, and 86.3%, respectively, after 12 days. The bacteria were cultured in a heterotrophic medium, and the pH was adjusted to 7.0. Microalgae were cultured in a constant-temperature light incubator at 12.5 μmol photons m−2 s−1 and 24.0 ± 1.0°C in BG-11 liquid medium for 7–8 days. Also, they studied antibiotic (Oxytetracycline (OTC) and Enrofloxacin (EFX)) removal using the algal-bacterial consortia system. Removal rates of Total Organic Carbon (TOC) and TP were 97.84–99.76% and 42.68–42.90% in the low-dose groups (OTC <5 mg L−1, EFX <1 mg L−1) after 10 days. C. vulgaris cultivated BG-11 culture medium, pH 7.34 ± 0.14 with the initial cell ratio of C. vulgaris: B. licheniformis was 1: 3 at temperature 28 ± 1°C, with 12 h light/12 h dark circulation at light intensity 120 μmol photons m−2 s−1 (Wang et al., 2022).
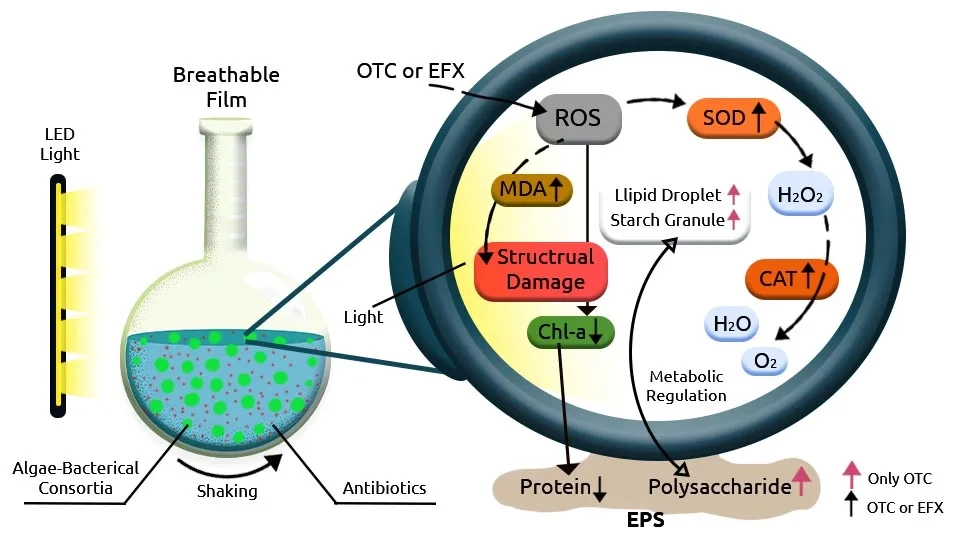
Jaiswal et al. (2019) investigated the toxicity of Organophosphate (OPs) insecticides Monocrotophos (MCP) and Chlorpyrifos (CLS) on Plant Growth-Promoting (PGP) properties inoculated by Microbacterium hydrocarbonoxydans, Stenotrophomonas rhizophila, Bacillus licheniformis and Bacillus cereus. These pesticides were removed at the rate of 54–90% after 5 days. Alcántara et al. (2015) supported an efficient removal of total organic carbon (86-90%), inorganic carbon (57–98%), and TN (68–79%) during synthetic wastewater treatment at a hydraulic and sludge retention times of 2 days and 20 days, respectively using algal-bacterial symbiosis. Temperature and magnetic agitation of the photobioreactor were maintained constant at 24 ± 1°C and 300 rpm with light intensity 400 ± 51 µE/m2·s at the outer wall of the photobioreactor and pH at 7.8 ± 0.1. Juárez et al. (2018) evaluated methane production from pretreated and raw mixed microalgae biomass grown in pig manure. The microalgae species Tetradesmus obliquus (29%), Tetradesmus lagerheimii (26%), Desmodesmus poliosis (16%), Aphanothece Saxicola (11%), Chlorella vulgaris (5%), Scenedesmus magnus (4%), Parachlorella kessleri (3%) removed pig manure with rate of 80–100% after 40 days. The systems were incubated in a rotatory shaker at 50°C and 120 rpm for 60 min with a pH of 11.5.
Microalgae-bacteria consortia, including Chlorella vulgaris, Chlorella kessleri, Pseudomonas pseudoalcaligenes, and Bacillus licheniformis, have been studied for their potential in wastewater treatment and various pollution remediation. Chlorella vulgaris and Chlorella kessleri are microalgae species known for their ability to remove nutrients such as nitrogen, phosphorus, and other pollutants from wastewater. Pseudomonas pseudoalcaligenes and Bacillus licheniformis bacteria can also play a role in breaking down organic carbon and some of the 9 pollutions in this paper. When these microalgae and bacteria are combined in a consortium, they can work synergistically to degrade pollutants and remove ECs from wastewater. The microalgae can utilize the nutrients present in the wastewater for growth, while the bacteria can help break down organic compounds and assist in nutrient cycling. However, the effectiveness of a specific consortium will depend on various factors such as the composition of the pollutants, environmental conditions such as temperature, pH, salinity, light intensity, etc., and interactions between the different species. Further research and optimization may be needed to determine the optimum conditions for high removal efficiency.
4. SS-PM Microalgae-Bacterial Combination
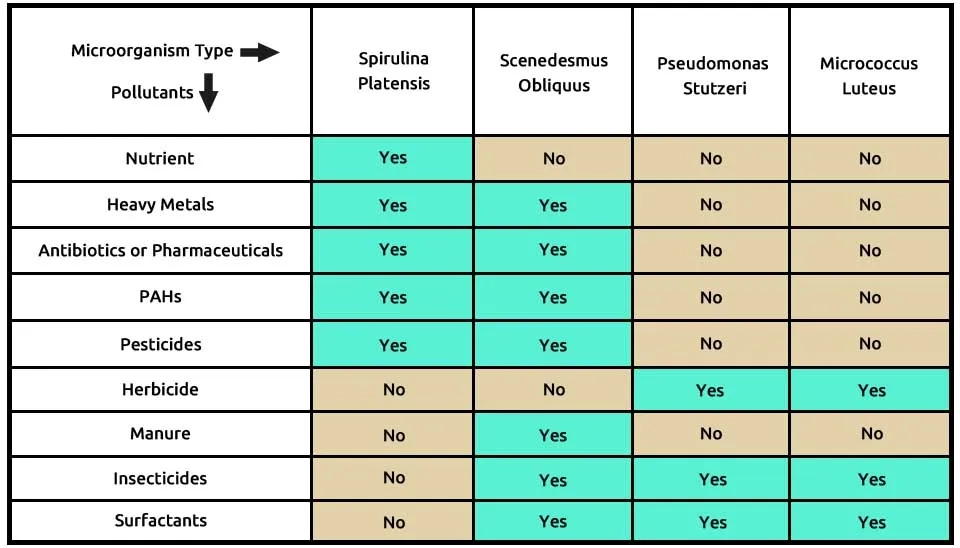
The fourth suggested combination consisted of the two microalgae, including S (Spirulina platensis) and S (Scenedesmus obliquus), two bacteria P (Pseudomonas stutzeri ) and M (Micrococcus luteus) that completely remove these 9 contaminants, according to Table 6. Spirulina platensis is able to remove many pollutions, such as nutrients, heavy metals, antibiotics or pharmaceuticals, PAHs, and pesticides. Scenedesmus obliquus helps it in heavy metals, antibiotics or pharmaceuticals, PAHs, pesticides, manure, insecticides, and surfactants removal. Herbicides, insecticides, and surfactants are completely removed by two bacteria, Pseudomonas stutzeri and Micrococcus luteus. The composition of microalgae, including Spirulina platensis and Scenedesmus obliquus, two bacteria, including Pseudomonas stutzeri and Micrococcus luteus, in the removal of 9 pollutants of ECs, is presented in Table 5.
Fu et al. (2023) adopted Spirulina platensis microalgae as the target algae stain for synthetic mariculture wastewater treatment with maximum nutrient removal rates 86.5% of TN (to 3.4 mg/L), 98.1% of TP (to 0.1 mg/L) and 95.8% of dissolved organic carbon (DOC) (to 5.5 mg/L) after 30-day granulation process. The target microalgae S. platensis grown in 1.0 L flasks under a complete cycle of 12:12 h light/dark periods at a controlled temperature of 25 ± 0.5°C, light intensity of 80 µmol m-2 s-1 at the flasks’ surface and the growth Zarrouk Medium (ZM). Abdel-Razek et al. (2019) used a consortium of microalgae, including Chlorella vulgaris, Scenedesmus quadricuda, and Spirulina platensis, to remove the organophosphate pesticide malathion, and the heavy metals cadmium, nickel, and lead from water samples taken from varying combinations of urban wastewater and agricultural drainage water. They founded a consortium of various microalgae that can be effective in remediating the pesticide malathion and the heavy metals cadmium, lead, and nickel from wastewater with a removal efficiency of 88–95% after 28 days. Strains were incubated at 23 ± 1°C in a culture room under a 24-hour photoperiod by using a 25-watt bulb in each aquarium and the addition of 1.5% agar to BG-11 medium. Ibrahim et al. (2014) evaluated using microalgae-bacteria consortia for wastewater treatment (91%) of organophosphorus pesticide malathion after 20 days. Amongst the various strains of microalgae, only Nostoc muscorum strain, with its capability to utilize malathion as a sole phosphorous source, is considered an inexpensive and efficient biotechnology for remediation of organophosphorus pesticide from contaminated wastewater. The culture flasks were kept under continuous illumination provided by daylight fluorescent tubes with an average light intensity of 40 µE m-2 s-1 maintained constantly during the experiment. The flasks were incubated in a culture room at 28 ± 1°C under continuous shaking of 80 rpm and BG-11 and Zarrouk medium.
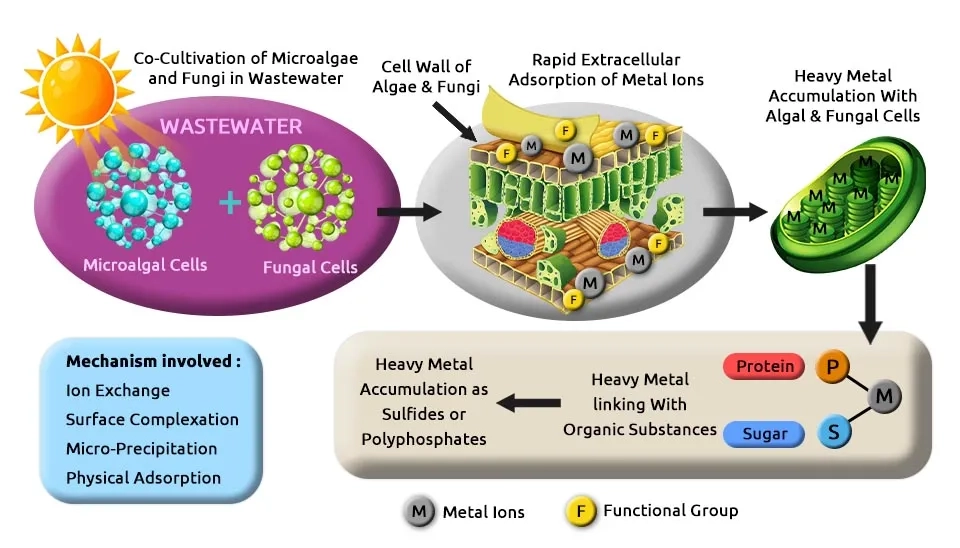
Al-Homaidan et al. (2015) used dry biomass of the microalga Spirulina platensis as a biosorbent for the removal of cadmium ions (Cd2+) from aqueous solutions. They showed that it reached the highest percentage of removal (87.69%) at pH 8, 2 g of biosorbent, 26°C, and 60 mg/l of cadmium concentration after 90 min of contact time. S. platensis was rinsed with deionized water and dried overnight in an oven at 80°C. Zhai et al. (2015) recovered nutrients and treatment of wastewater using wastewater as a medium to cultivate microalgae. They cultivated Spirulina platensis in Synthetic Municipal Wastewater (SWW) with 8.8–8.9 for pH, the light intensity of 3300–3400 lx in the daily illumination time for 12 h when the temperature 25 ± 1°C with the air-bubbling of 0.5 vvm. Spirulina platensis removed TN and TP were 92.58 and 94.13%. Janoska et al. (2018) showed a novel liquid foam-bed photobioreactor as an innovative technology for microalgae production. They used a foam stabilizing agent for an optimal surfactant. They compared ten different surfactants (non-ionic, cationic, and anionic) and two microalgae genera, Chlorella sorokiniana and Scenedesmus obliquus, on the above-mentioned criteria. For all surfactants tested, the microalgae concentration is reduced in the foam phase compared to the liquid phase except for the cationic surfactant Cetyltrimethylammonium Bromide (CTAB). Strains cultivated in M8a media with 250–300 mL shake flasks placed in an orbital shaker in an incubator and temperature 25–37°C, 120–454 µmol m-2 s-1, 80–120 rpm and 2.5–4% CO2.
Tang et al. (2012) degraded crude oil using one artificial microalgal-bacterial consortium, including Scenedesmus obliquus and four oil component-degrading bacteria with Sphingomonas GY2B, Burkholderia cepacia, Pseudomonas GP3A, and Pandoraea pnomenusa. Results showed the consortium could completely eliminate alkanes, alkyl cycloalkanes, and alkylbenzenes PAHs in 10 days, 7 days, and 7 days, respectively. Microalgal-bacterial cultures were inoculated into 100 ml flasks containing 30 ml BG-11 medium. Oseberg crude oil was added as the initial concentration was 0.3% (v/v). Flasks were incubated at 25 ± 1°C with constant shaking at 150 rpm under a light-dark regime of 14:10 and illumination of 165 µmol m-2 s-1. Li et al. (2021) used the co-culture of Scenedesmus obliquus microalgae and specific Bacillus megaterium bacteria as conducive to improving wastewater treatment efficiency. They found that the C/N/P ratio in biogas slurry was 106/16/1 and the initial microalgae-bacteria inoculation ratio was 9:1, the co-culture attained the maximum COD, TP, and ammonia nitrogen (NH4+-N) removal rates of 85.98 %, 81.03 %, and 65.48 %, respectively. The strains were cultivated in BG-11 media at 25 ± 2°C under cool-white fluorescent light illumination at 45 µmol m-2 s-1 with the light-dark cycle of 14 h:10 h.
Rambaldo et al. (2022) studied the long-term assessment of green technology (1–4 L/day) based on a Membrane Bioreactor (MBR) technology-based system, using a Photobioreactor (PBR) containing immobilized microalgae–bacteria (e.g., genus Phenylobacterium, Sphingomonadaceae, and Caulobacteraceae, Scenedesmus obliquus or Acutodesmus obliquus, Tetradesmus, Chlorella, and Coelastrella) in Polyurethane Foam (PF) followed by a Cork Filter (CF) for removing nitrates, pesticides such as atrazine and bromacil, and antibiotics such as sulfamethoxazole and sulfacetamide from groundwater with hydraulic retention time (HRT) of 8 days. Membrane Filtration Technology is a crucial component in achieving such high removal efficiencies They showed moderate effectiveness for removing nitrates (58%), with pesticides being the compounds most affected (reducing from 97 to 98% at an HRT of 8 days). The prototype was tested in a temperature-controlled room at 23 ± 5°C. The pH was monitored every week and maintained at around 7 throughout the experiment, with CO2 injected at 0.03% of the total water volume of the PBR (5.6 L); the light generated 105 µmol m-2 s-1. Saikia et al. (2005) completely degraded β-cyfluthrin pesticide using Pseudomonas stutzeri at 20 days. They used a Mineral salt medium for the isolation of bacterial strain at a pH of 7.0. After mixing well, the preparations were incubated in Petri dishes at 28°C for 30 days. Eniola. (2012) used various consortia of pure cultures of Alcaligenes odorans, Citrobacter diversus, Micrococcus luteus, and Pseudomonas putida for biodegradation (86%) of Linear Alkylbenzene Sulfonate (LAS) surfactant after 16 days. Bacterial strains cultivated in LAS Mineral Medium (LMM), pH 7, room temperature (28 ± 3°C) on an orbital shaker at 100 rpm. According to research, microalgae-bacterial consortiums in this composition can grow together in the environment. However, the outcome of their interactions depends on factors such as nutrient availability, competition for resources, and production of antimicrobial compounds. In some cases, specific bacterial strains outcompete others, while in other cases, they may coexist peacefully. It ultimately depends on the particular conditions of the environment in which they are growing.
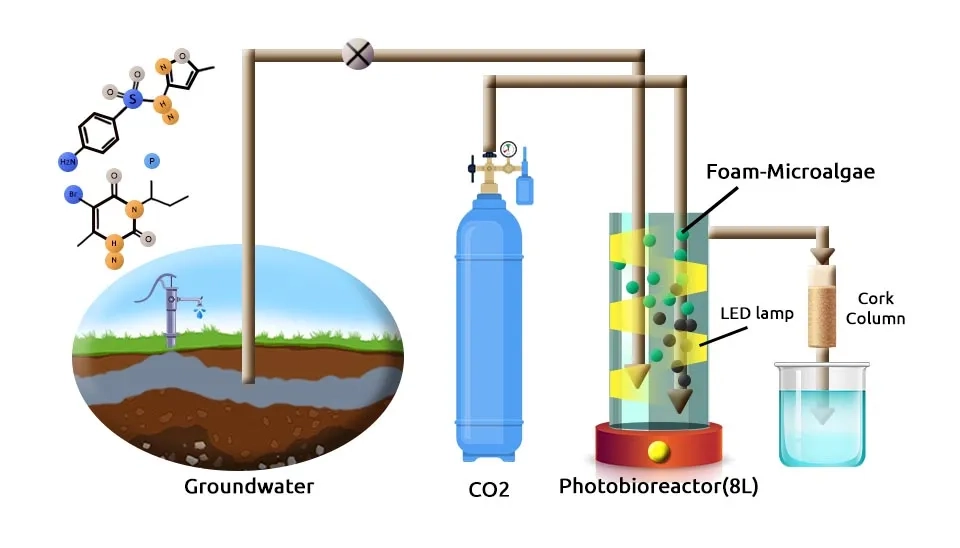
Conclusion
The factors that affect the algal-bacterial systems and have a huge potential for wastewater treatment in a sustainable way are pH, light intensity, light/dark cycle duration, temperature, nutrients, and mixing conditions. This article will explore the potential of microalgae-bacterial consortia for the simultaneous removal of 9 emerging contaminants from wastewater. The author suggests this microalgae-bacteria consortia composition for contamination removal should be investigated for the adapting cultivation conditions and microalgae and bacteria species. Microalgae-bacterial consortia systems are likely to be more efficient in removing emerging contaminants than conventional treatment systems. The increase in the removal rates can be attributed to the possible symbiotic interactions in the microalgae-bacterial consortium and additional photodegradation due to light, temperature, medium type, pH, etc. To implement microalgae-bacterial consortium systems at field-scale, more emphasis should be given to i) the selection of capable algal-bacterial strains, ii) optimization of light illumination as it is the main limiting factor for photosynthesis, iii) modeling the systems in the long run, and optimizing the design and operational parameters iv) life cycle analysis and techno-economic feasibility to assess the reliability of these treatment systems. According to the research conducted, these proposed compositions of the microalgae-bacteria consortium have removed all pollutants from that family examined in the research, and more detailed tests are needed to remove all the pollutants. Biomass conversion into bioproducts such as biodiesel, bioelectricity, biohydrogen, and bioethanol require more research.