
One of the main industrial pollutants is caused by discharging wastewater (Nabarlatz et al., 2012). Adsorption is known to be an efficient and effective technique used to adsorb or retrain the contamination (Lei et al., 2011). Activated carbon is one of the commonly employed materials as an adsorbent for applications in the liquid phase due to its high pore volume and pore size distribution. However, the high cost of carbon materials is a main drawback that can be minimized using the regeneration of activated carbon which increases the number of times it can be used (Dabrowski et al., 2005; Mourão et al., 2008).
The methods of activated carbon regeneration have been explored by scientists all over the world and many ways have been put forward, such as chemical regeneration, solvent regeneration, etc. (Guo et al., 2011). In this paper, we discuss different methods for the regeneration of activated carbon as well as the recently developed and novel methods of activated carbon regeneration.

1. Regeneration of Activated Carbon
Activated carbon, which is composed of a highly porous structure produced by physical or chemical activation, is a common solid-phase adsorbent. The activation process can increase the porosity of carbon, which makes it effective at adsorbing small molecular compounds. For treating the exhausted or saturated carbon, some options are available: (1) disposal; (2) regeneration; and (3) reuse with the necessary pre-treatment (usually referred to as reactivation) (Nahm et al., 2012). In particular, regeneration is considered a cost-effective method to prolong the carbon life rather than disposal (like landfill) or carbon replacement. Since these two methods have some specific drawbacks in terms of their economic and environmental impacts, regeneration is a preferable option among scientists (Dotto and McKay, 2020).
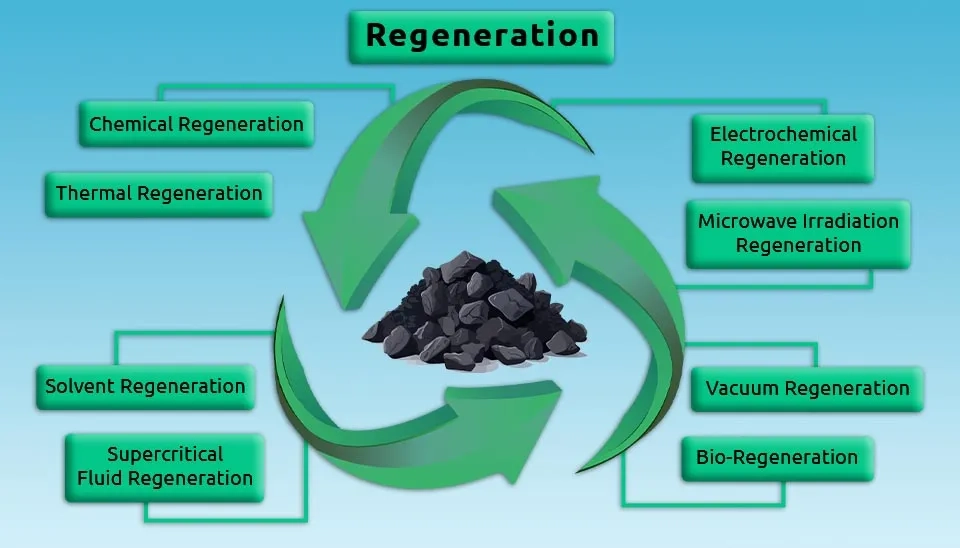
2. Regeneration of Activated Carbon Methods
Regeneration has been increasingly regarded as a key issue in adsorption practice in water treatment industries, and developing a primitive, new, alternative, and eco-friendly method for regeneration of activated carbon is needed worldwide. The fact that the regeneration process may produce undesirable by-products or alter the carbon characteristics has been a major concern for this method (Marsh and Rodríguez-Reinoso, 2006).
In recent decades, different ways for activated carbon regeneration have been used due to the application, including chemical regeneration, thermal regeneration, solvent regeneration, electrochemical regeneration, etc. Unfortunately, in most regeneration methods, the economy is proven to be a major barrier to the extensive application of these methods in civil and environmental engineering (Lai et al., 2007; Zhang et al., 2010). In the following, different regeneration methods for activated carbon, as well as their advantages and disadvantages, are reviewed.
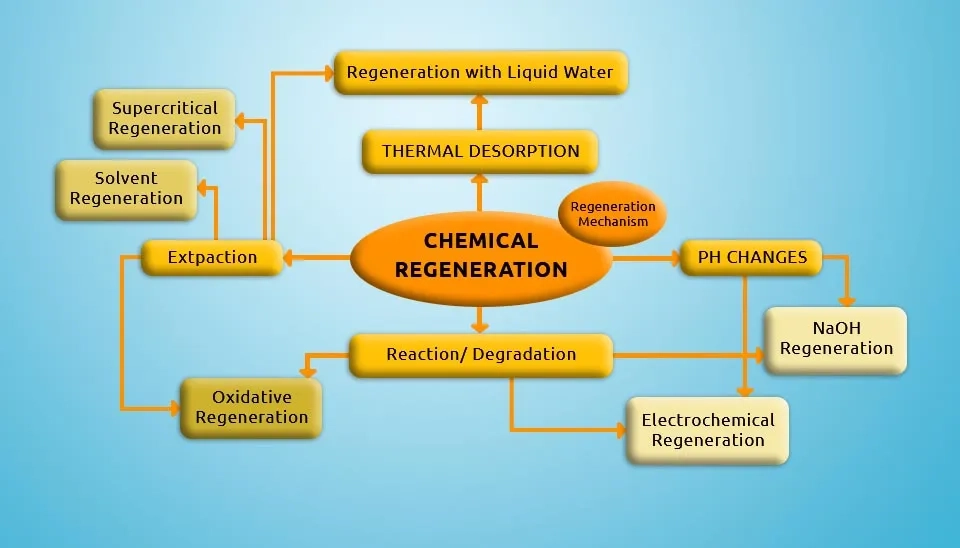
2.1. Chemical Regeneration
Chemical regeneration is related to changing the nature of the adsorbed compound through acid-base or redox chemical reactions in conditions where chemical agents affect the composition of adsorbed compounds or disturb the equilibrium of the adsorption process. Some chemical regeneration methods include regeneration with liquid water, NaOH, and solvents, supercritical regeneration, and electrochemical regeneration (Larasati et al., 2021).
The general function of chemical regeneration depends greatly on the operating variables like solution temperature and the regenerant solution selected for the process. The perfect performance of chemical regeneration can be achieved at a moderate to high temperature > 50 °C, and at room temperature, the process can not easily occur (Larasati et al., 2021). Some characteristics should be taken into account when choosing the regenerant, such as their nature and combination of adsorbates, solubility, molecular weight, hydrophobicity, toxicity, and boiling point (Martin and Ng,1984).
Ease of operation, not of carbon attrition, minimizing the downtime for carbon transportation, and capability of regenerant and adsorbate recovery are known to be several advantages of chemical regeneration over other methods. In comparison with thermal regeneration, chemical regeneration is more appropriate and economically feasible for smaller-scale industrial applications. However, besides the positive aspects, the potential waste chemical which requires further treatment, is still a major concern and should be considered (Dutta et al., 2019). In addition, for the chemically regenerated carbon, a rinsing step is needed to remove the remaining chemicals on the carbon. This step may represent a large clean water consumption or when organic solvents are used as regenerant, hot air or hot water is used to purge (Cooney et al., 1983).
Table 1. Regeneration efficiency of chemical regeneration using inorganic solutions and solvents at room temperature (20 – 25 ◦C) for activated carbon (Larasati et al., 2021)
Regenerant solution | RE % | Time (h) |
|---|---|---|
HCl 10% (w/w) | 53 | 24 |
NaOH 6 M | 36 | 2 |
NaOH 1 M | 92 | 12 |
Methanol | 51 | 2 |
Benzene | 42 | 2 |
DMF | 92 | 0.25 |
Ethanol | 78 | 24 |
Formic acid | 92 | 24 |
Acetic acid | 17 | 2 |
Acetone | 94 | 24 |
CTAB 10-30 cmc | 12-30 | 24 |
cmc: Critical micelle concentration
RE: Regeneration Efficiency
w/w: Weight concentration (weight per weight)
DMF: Dimethylformamide
CTAB: Cetyltrimethylammonium Bromide
M: Molarity
CTA: If you want to supply regenerant solutions for activated carbon regeneration, click here.
2.2. Thermal Regeneration
Thermal regeneration that uses hot gasses is the most widely used and common industrial-scale regeneration method for spent carbon. General thermal regeneration methods include thermal swing adsorption, steaming, electrothermal swing adsorption, and microwave regeneration.
Two main processes are involved in thermal regeneration, including pyrolysis and oxidation. To remove the moisture, spent carbon should be dried at 105 ℃ and then it is ready for regeneration (Samonin et al., 2013; Zanella et al., 2014). Although thermal regeneration is proven to be the most practical method, it has certain disadvantages that are still an issue:
(a) It causes 5-10% carbon capacity loss due to oxidation and attrition
(b) The high energy cost of heating the activated carbon to approximately 800 ℃ (San Miguel et al., 2002)
(c) The contaminants shift to the air phase
(d) For small applications, an extremely high cost is required.

2.3. Solvent Regeneration
For methods with reversible adsorption, such as recycled wastewater containing precious metals and high organic concentration wastewater treatment, solvent regeneration is mainly applied. Solvent regeneration has several advantages including the following: (1) this method easily recovers useful substances from the regeneration liquid, (2) it does not cause damage to the porous structure, (3) the method can be implemented easily in practical production processes, (4) the loss of activated carbon occurs less than other regeneration methods (Pérez-Gregorio et al., 2010). There are also severe disadvantages when using solvent regeneration, including (1) solvents usually make most pollutants desorbed; (2) since the pores in the activated carbon can become air-logged easily, regeneration does not occur completely; (3) it is vital to find an efficient method for recovering organic solvents free of pollutants; (4) the economic costs increase by using organic solvents; (5) these chemicals are pollutant and mostly toxic; and (6) solvent regeneration is not an environmentally friendly method (Martin and Ng,1984).
Guo et al. (2011) showed that using methylene dichloride, n-pentane, and ethyl ether organic solvents for regenerating activated carbon can efficiently remove refractory organic compounds that are adsorbed on the activated carbon’s surface during the wastewater treatment process. In addition, n-pentane enabled multiple regenerations of activated carbon exhausted in wastewater treatment, reduced regeneration costs, displayed excellent regeneration efficiency, and gradually increased adsorption ability.

2.4. Supercritical Fluid Regeneration
Supercritical fluid regeneration is developed from the normal chemical generation method. Carbon dioxide (CO2), which can extract and dissolve organic compounds, is known to be the most commonly used fluid. Salvador et al. (2013) showed that supercritical fluid regeneration is very effective in spent carbon regeneration several times. They also found that this technique can completely regenerate the carbon saturated by phenol in 10 min, which potentially makes this method very rapid (Salvador et al., 2013).
The remarkable advantage of this regeneration method is that, unlike organic solvents, using fluids (CO2 and water) does not cause environmental issues. However, the main disadvantage of this method is the high capital cost required for infrastructure and most of the studies have been done at a laboratory scale, where its efficiency has been approved (Liang et al., 2012).
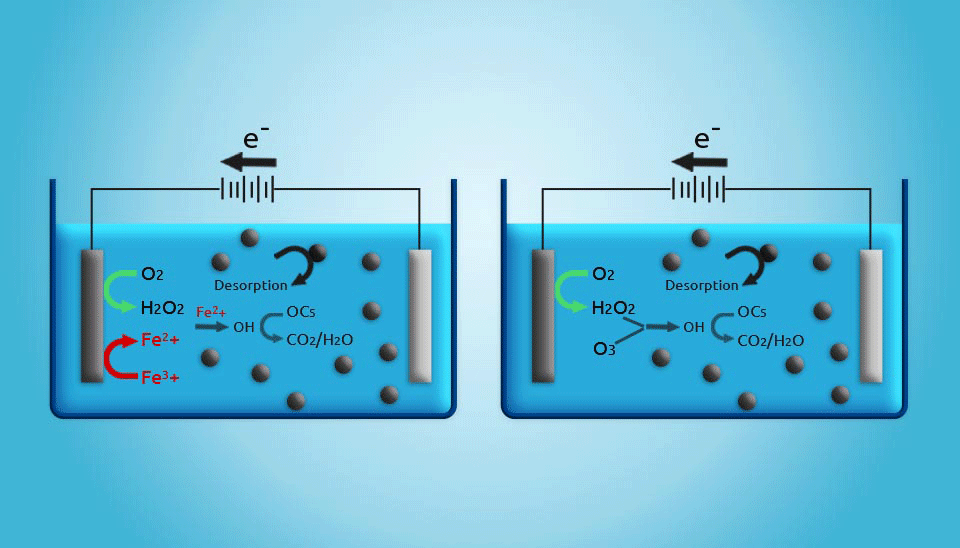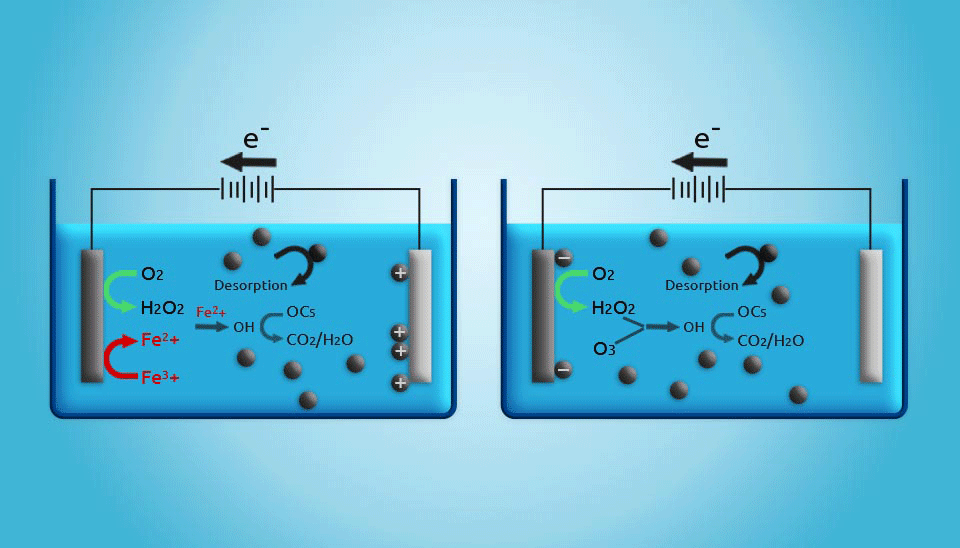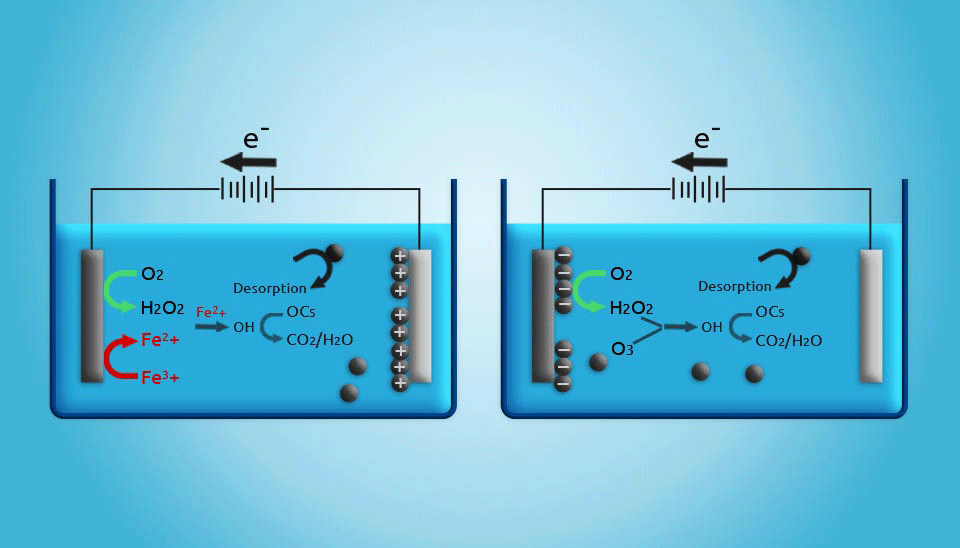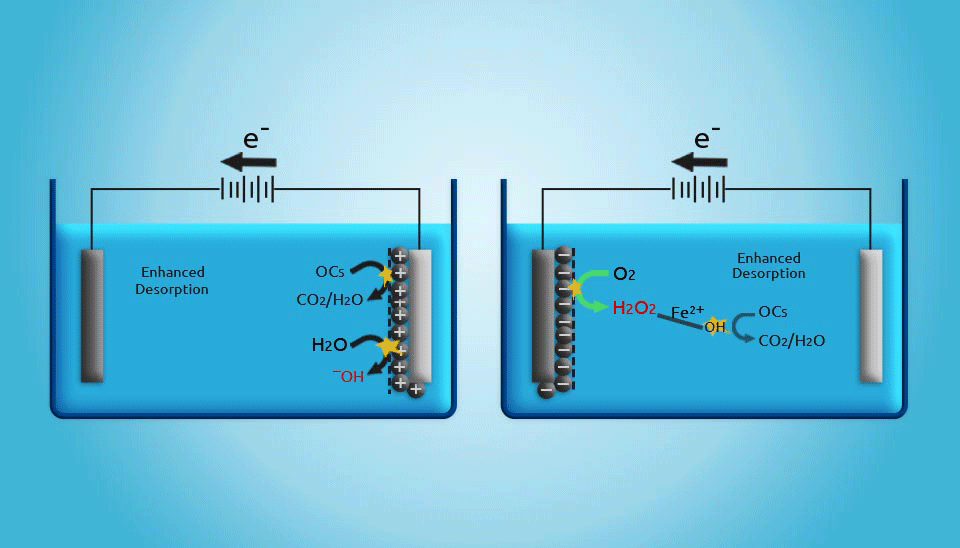
2.5. Electrochemical Regeneration
Electrochemical regeneration is mainly based on the process that occurs when a saturated adsorbent is exposed to an electrical current in an electrochemical cell. Even though the regeneration efficiency of electrochemical units is not as high as that achieved by thermal regeneration, it should be noted that in thermal regeneration, up to 20% of the activated carbon mass will be lost. Therefore, electrochemical regeneration is more cost-effective (Clements and Haarhoff, 2004; Miguel et al., 2001). Furthermore, the costs of electrical power for electrochemical regeneration are estimated to be lower than those for thermal regeneration in small wastewater treatment systems. Generally, although electrochemically activated carbon regeneration has shown to be a promising method, the development of an adequate reactor that is capable of regenerating large amounts of activated carbon is still a major challenge (Narbaitz and Karimi-Jashni, 2012).
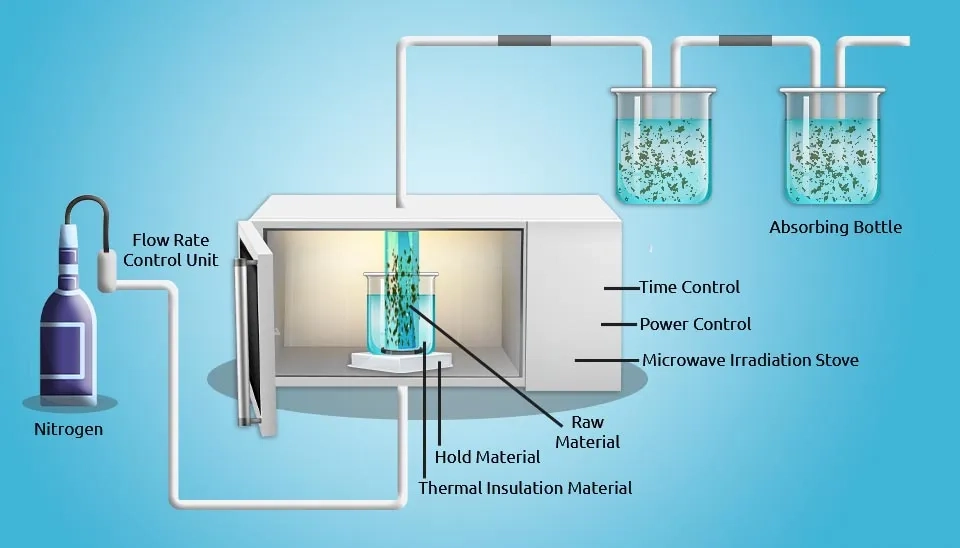
2.6. Microwave Irradiation Regeneration
Microwave irradiation has become an important method of activated carbon regeneration in recent decades due to its ability to molecular-level heating. Several studies have been carried out that show promising results about the application of microwave irradiation in activated carbon regeneration (e.g., Deming et al., 2011; Guido et al., 2008; Bermudez et al., 2012). For economic purposes, this method has been widely used in various industries (Remya and Lin, 2011).
In comparison with conventional regeneration methods, microwave irradiation offers many advantages. For Example, the activated carbon capacity can be rescued by loading metal on activated carbon. In this condition, microwave irradiation has a beneficial impact on modified activated carbon regeneration and can increase the adsorption capacity after cycles of regeneration (Ania et al., 2007; Yuen and Hameed, 2009).
2.7. Bio-Regeneration
The general mechanism of bio-regeneration is based on microorganism metabolism. This includes: (1) the microbes consume the adsorbed compound (mostly organic matter) as their energy source, (2) microbes create exo-enzymes that can diffuse to the meso- and micropores of carbon and cause hydrolysis, which results in lower adsorption affinity products that are easily desorbed from the biofilm and carbon pores; (3) the concentration gradient that develops because of the microbial degradation process, which leads to desorption process (Nath and Bhakhar, 2011).
The beneficial aspects of bio-regeneration are its simplicity, low level of investment, and environmentally friendly properties. Bio-regeneration offers a low-cost activated carbon regeneration technique, but its low efficiency is known as one of the drawbacks. The other disadvantages including the slow desorption rate (regeneration times longer than two days) and the presence of harmful products and microbes make this method unsuitable for the regeneration of activated carbon, especially for drinking water treatment applications (Hutchinson and Robinson, 1990; He et al., 2012).
2.8. Vacuum Regeneration
In gaseous flow applications, vacuum regeneration is very practical to desorb and recover highly volatile compounds from the spent carbon. To date, no investigations have been carried out in the field of vacuum regeneration for liquid phase adsorption-desorption processes. In this regeneration method, adsorbent and adsorbate interactions are affected by the decrease in the atmospheric pressure in carbon beds (Salvador et al., 2015).
In some aspects, vacuum regeneration is similar to thermal regeneration, since an inert gas like nitrogen is used in this process. Using the inert gas does not leave moisture on the carbon’s surface, which means that a drying process will not be required when regeneration is done (Ramalingam et al., 2011).

3. Conclusion
Regeneration of activated carbon has been a desirable and widely used option in groundwater treatment, drinking water treatment, and industrial wastewater treatment systems. Generally, methods for activated carbon regeneration are classified into four main groups based on the agents involved in regeneration and the mechanism: chemical regeneration, thermal regeneration, microbiological regeneration, and vacuum regeneration. Removal of pollutants and recovering the original adsorption capacity of the activated carbon is the main purpose of regeneration. However, regeneration is not always economically feasible due to the high costs of facilities, and in some methods, it may lead to significant mass loss or may alter the porous structure of the activated carbon. Although many scientists have done extensive research on the various activated carbon regeneration methods, thermal regeneration is often used in industries. Further work on different methods for activated carbon regeneration is required to properly understand their mechanism, to better use them for water and wastewater industries, and also to develop new and improved existing regeneration methods to provide efficient, cost-effective, and environmentally friendly alternatives.
