Strategies such as seawater desalination and water reuse have been implemented to meet the growing demand for fresh water. One of the best methods for producing high-quality water is membrane technology. To overcome the safe drinking water shortage, which is one of the main issues all around the world, membrane-based solutions have become increasingly popular, including reverse osmosis, ultrafiltration, membrane distillation, electrodialysis, etc. Moreover, membrane-based processes are also used for wastewater treatment as well as the treatment of brackish water (Jiang et al., 2017).
At present, several obstacles still need to be improved, and one of these bottlenecks is the fouling of membranes, which is somehow inevitable in membranes. After the commercialization of membranes, fouling has been one of the main topics of research and studies (Maqbool et al., 2019).
Membrane fouling leads to economic expenses because of an increase in feed pressure, a reduction in water permeability, membrane replacement, and multiple Cleaning In Place (CIP) operations. Membrane fouling causes more than 24% and 11% of operational expenses in the reverse osmosis and ultrafiltration plants, respectively. Membrane replacement and an increase in energy demands are the main factors in the increase in operational expenses (Jafari et al., 2020).
In this paper, types of membrane fouling, with a focus on recent developments, are reviewed.
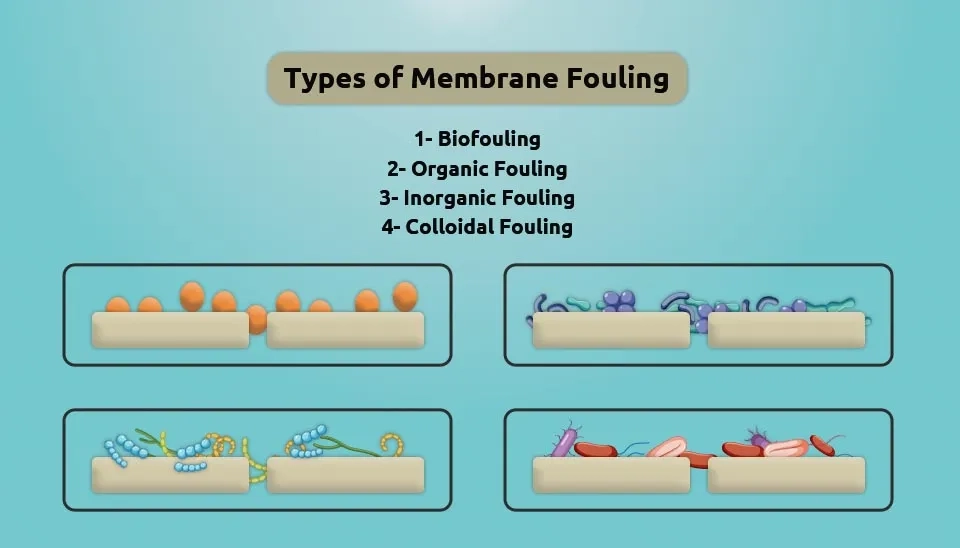
Membrane Fouling | The shape of fouling | Cause | Sign |
Biofouling |  | The process of microorganism adhesion and proliferation on the membrane surface | Strong odors and mold |
Organic Fouling |  | The organic matter present in feed water adsorbs on the membrane surface and forms irreversible fouling | Increase in pressure-demand |
Inorganic Fouling |  | The deposition of inorganic substances on the membrane surface or inside the membrane pores | Increase in pressure-demand |
Colloidal Fouling |  | The process of membrane fouling is caused by colloids/particles that are deposited on the host material | Poor water quality in treated water as a result of defects in the pretreatment step. |
1. Membrane Fouling
The deposition of material like salts and solids on the external of internal surface of the membranes is often referred to as fouling. This deposition decreases the efficiency of transmembrane pressure, which leads to a reduction of permeate flux (Baker, 2004). Fouling is typically divided into two main categories: internal and surface fouling. Due to the deposition of minerals, biofilm development, and Cake layer, fouling may also occur on the external surface of membranes. Membrane fouling ultimately leads to flux reduction, higher operation pressure, frequent chemical cleaning, and shorter membrane life (Jiang et al., 2017).
2. Types of Membrane Fouling
Fouling is caused by to bio, organic, inorganic, and colloidal foulants. Membrane fouling leads to an increase in energy demands, additional pretreatment, membrane cleaning, foulant removal, and maintenance, a reduction in permeate quality and productivity, as well as increased operation costs (Kochkodan et al., 2014). In the following, the key elements and different membrane fouling types are discussed in detail.
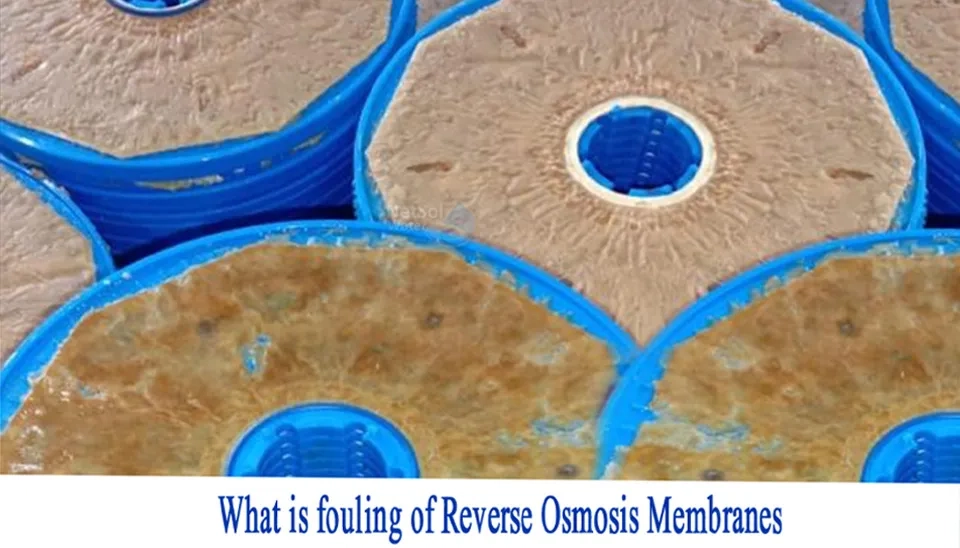
2.1. Biofouling
The process of microorganism adhesion and proliferation on the membrane surface is called biofouling. The bacteria and the Extracellular Polymeric Substances (EPS) are two key components of biofilms that were excreted during the metabolism process (Yu et al., 2016). Bacterial communities are highly diverse and distinctive in marine environments, and the common ones are firmicutes, cyanobacteria, proteobacteria, and Bacteroides (Belila et al., 2016). Depending on the water environment and bacteria community, EPS may contain different substances, but they are mainly made up of proteins, glycoproteins, polysaccharides, lipoproteins or lipids, and nucleic acids (Matin et al., 2011). From another point of view, biofilm formation can be divided into three phases in terms of bacteria mobility: bacteria attachment, reproduction, and detachment.
Biofouling is widely regarded as one of the most formidable fouling types and is more complex than other membrane fouling types (Hibbs et al., 2016). It is difficult to eliminate membrane biofouling by pretreatment methods, unlike other types of membrane fouling. Biofouling is caused by microorganisms, which can easily grow and multiply. Therefore, one hundred percent of the bacteria must be removed by pretreatment. Otherwise, the remaining organisms can grow on the membrane surface and lead to membrane fouling.Fig. 3 Membrane biofouling

2.2. Organic Fouling
Organic fouling is caused by organic matter that is present in feed water. The organic matter adsorbs on the membrane surface and forms irreversible fouling. Organic matter typically consists of humic substances, polysaccharides, proteins, lipids, nucleic acids and amino acids, organic acids, and cell components (Jeong et al., 2016). Generally, organic fouling can be divided into three types: Natural Organic Matter (NOM) (for surface water or seawater), Algal Organic Matter (AOM), and Effluent Organic Matter (EfOM) (for wastewater) (Kim & Dempsey, 2013).
Organic fouling leads to a significant flux decline in membranes, which is hard to eliminate due to the complex structure formed by dissolved organic matter in combination with other substances (Ding et al., 2016). An important factor for membrane fouling is the molecular weight of organic matter (Teixeira & Sousa, 2013). In addition, it is more difficult to remove organic matter with low molecular weight by conventional pretreatment techniques like coagulation (Fabris et al., 2008).

2.3. Inorganic Fouling
Inorganic fouling or scaling occurs as a result of the precipitation of salts over the membrane surface. Inorganic fouling is actually the deposition of inorganic substances on the membrane surface or inside the membrane pores (Henthorne & Boysen, 2015). When feed water comes into contact with the membrane, the water passes through the membrane while salts leave behind. Salts start to precipitate on the membrane surface, and scaling forms when the concentration of salts increases compared to the solubility product. Scale deposition along with the cake layer does not allow water to contact the membrane, which gradually leads to membrane degradation, flux reduction, production loss, and higher operating costs (Sim et al., 2018).
Statistical analysis showed that in the last 10 years, scientists and researchers have tended to study calcium sulfate and calcium carbonate more than other foulants, which indicates their significant role in causing inorganic fouling. Other common inorganic foulants consist of calcium phosphate, barium sulfate, aluminum silicate, and so on. Inorganic fouling on membranes could be affected by a number of factors such as membrane properties, compositions, features of the feed water, and operating conditions (Rabie et al., 2001).

2.4. Colloidal Fouling
Colloids are fine suspended particles that vary from a few micrometers to some nanometers in size (Al-Amoudi & Lovitt, 2007). The particle size has a major influence on the colloidal fouling deposition. Colloidal fouling is a process of membrane fouling caused by colloids or particles that are deposited on the host material. Typically, colloidal foulants can be divided into two groups: inorganic foulants and organic macromolecules. Aluminum silicate minerals, silica, and iron oxides/hydroxides are the common inorganic foulants in natural water, while materials like proteins, polysaccharides, and some natural organic matter are the main organic macromolecules in the water (Tang et al., 2011). It is reported that inorganic pollutant concentrations of more than 0.05 mg/l for iron oxide, 1 mg/l for silica, and 0.02 mg/l for hydrocarbons would cause colloidal fouling in RO membranes. An increase in pH, temperature, and TDS will multiply this kind of fouling (Maqbool et al., 2019).
Colloidal fouling improves the fouling propensity when combined with inorganic and organic foulants (Qin et al., 2018). A number of factors, such as colloid size, shape, and interaction with ions of the colloids, may have an impact on colloidal fouling (Buffle et al., 1998). Specific interactions between foulant ions and membrane ions could have a significant effect on membrane fouling.

3. Conclusion
To address the world's water shortage issues, membrane filtration technologies have become very important for water reuse and desalination. However, membrane fouling has become a major challenge and an inevitable problem for membrane-based water treatment methods. Biofouling, organic fouling, inorganic fouling, and colloidal fouling are the main types of membrane fouling as discussed in this article which can occur depending on operation conditions, feed water quality, and membrane characteristics. The overall process efficiency and economics are adversely affected by membrane fouling. Generally, membrane fouling leads to flux decline, frequent chemical cleaning, higher operating pressure, and shorter membrane life. With suitable approaches and strategies, it is possible to control the membrane fouling.
If you know other types of membrane fouling, please comment for us.
4. Quiz
To test the material learned in this article, you are invited to answer the quiz raised from the text