
Globally, people consume thirty million tons of medications and produce over 50,000 distinct types of pharmaceuticals (Liu et al., 2020). Pharmaceuticals are physiologically active materials with a specific mode of action in both humans and animals. Pharmaceutical substances have frequently been reported to be found in drinking water, silt, sewage, and aquaculture effluent. Pharmaceutical enterprises, hospitals, animal waste, research involving medicinal compounds, and the release of expiredmedications into the environment are the main sources of pharmaceutical pollutants (Fig. 1). Hospitals are one of the main sources of medications released into the environment (Tiwari et al, 2016). Antibiotics and hormones were two classes of pharmaceuticals that were frequently found in natural water due to inadequate removal by wastewater treatment facilities (Padhye et al., 2014). Due to the mentioned facts, wastewater treatment in the pharmaceutical industry plays an important role in the contemporary world. The presence of pharmaceuticals in the aquatic environment causes a challenge to modern science. The most significant effect that the existence of pharmaceutical wastewater in marine ecosystems inducesis the emergence of antibiotic resistance, which can lead to a health emergency worldwide. It is necessary to recall that the impact of pharmaceuticals in the marine environment is not limited to resistance to antibiotics; pharmaceuticals can also affect the behavior and reproductive systems of aquatic organisms, with cascading effects on the entireecosystem (khan et al., 2023).
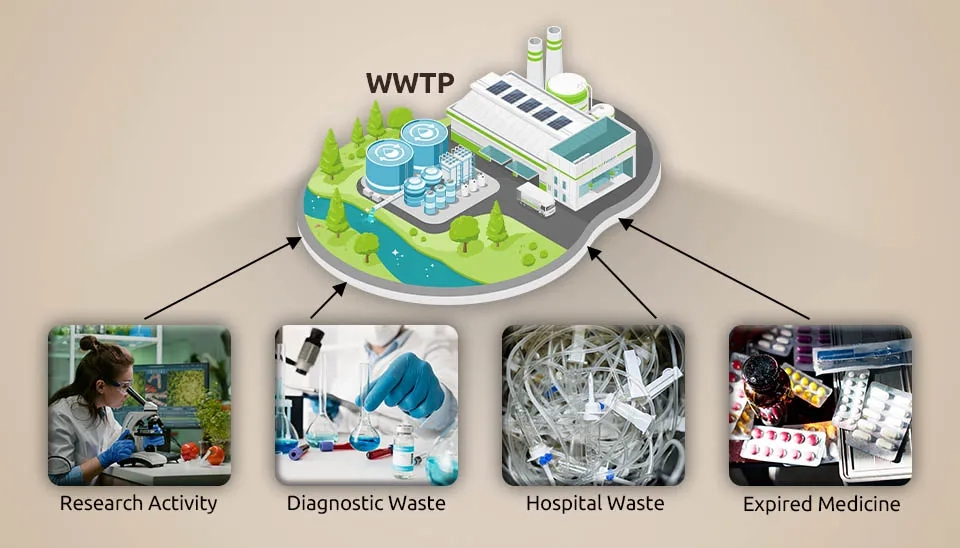
Table. 1. - Advantages and disadvantages of different Pharmaceutical Wastewater Treatment Methods
Treatment Methods | Sub Treatment Methods | Advantages | Disadvantages |
Physicochemical Processes | Coagulative Precipitation | - Effective Particle Removal - Wide Applicability - Reduced Turbidity - Simple Process - Can Be Combined with Other Treatments | - Chemical Dependency - Sludge Production - pH Sensitivity - Specificity to Contaminants - Risk of Overdosing - Not Suitable for Dissolved Solids |
Flotation | -High separation capacity | -High capital cost - High maintenance cost | |
Membrane Separation | -Fast and efficient -Low required Space | -Limited lifetime -High capital cost - High maintenance cost | |
Adsorption | -cost-effectiveness -excellent approach for removing organic pollutants | -require regeneration of the adsorbent | |
Electrolysis | -Low required Space -easy to operate -Consistent quality of the effluent -easy to control | -High energy consumption -High cost | |
Chemical Processes | Fenton | -Easy to operate -Low Capital cost | -Requires additional treatment |
Ozonation | -Fast and efficient -Lack of secondary pollutants -Low sludge production | -High reactivity -High capital cost -Toxicity -High maintenance cost | |
Photocatalytic Oxidation | -Lack of secondary pollutants -High capacity of oxidizing | -oxidative byproducts -high operation cost. | |
Biological Processes | Sequencing Batch Reactor (SBR) | -High efficiency in removing organic pollutants | -Hard to operate and control -requirement for more than one treatment unit -expense of providing aeration |
Upflow Anaerobic Sludge Blanket (UASB) | -High organic loading capacity -High efficiency In COD removal -Low energy demand | - Difficult to control -Limitation on nearly solids-free wastewater -Sensitivity to organic shock loads | |
Membrane Bioreactor (MBR) | -High quality of the effluent -High degradation rate | -Limitations in aeration -Membrane pollution -High Capital cost |
1. Pharmaceutical Wastewater
The key characteristics of pharmaceutical wastewater are (a) a high concentration of organic pollutants, a wide range in concentration, and complex composition (Gago-Ferrero et al., 2017); (b) a high BOD and COD value, as well as a significant difference between the two in wastewater (Segura et al., 2013); (c) a high concentration of NH₃-N and chroma (Fazal et al., 2015); and (d) a high concentration of suspended solids and salinity (Shi et al., 2014). As a result, Wastewater Treatment Plants (WWTPs) that employ traditional physicochemical and biological treatment methods have difficulty treating pharmaceutical effluent. Pharmaceutical wastewater's unique characteristics necessitate the use of special, advanced methods for treatment. So wastewater treatment in the pharmaceutical industry includes different treatment methods to tackle the issues that particular features of pharmaceutical wastewater may make.
2. Treatment Methods
There are advanced pharmaceutical wastewater treatment methods that are employed in treatment procedures that can be divided into three major categories: (1) physicochemical processes, (2) chemicalprocesses, and (3) biological processes. The most important methods for each of these categories are discussed in the following paragraphs.
2.1. Physicochemical Processes
Physicochemical processes that are commonly used for pharmaceutical wastewater treatment are coagulativeprecipitation, flotation membrane separation, adsorption and electrolysis
2.1.1. Coagulative Precipitation
Coagulation precipitation technology and its various types is being employed extensively worldwide due to its economic dependability, making it the preferred physical-chemical treatment technology. The stability of the colloids in pharmaceutical wastewater is reduced as a result of charge neutralization, bridging, and netting caused by the addition of chemical agents, which causes colloidal coalescence, bonding, and precipitation under the influence of gravity, resulting in pharmaceutical wastewater being separated. The simplicity of the process, its ability to be mixed with other treatment methods, and its effectiveness in particle removal are some advantages of the coagulation precipitation method. On the other hand, being sensitive to pH, large amounts of sludge leftovers, and low function with dissolved solids are some drawbacks of this method. Fig. 2 shows how the process of coagulation works (Yuan et al., 2016).

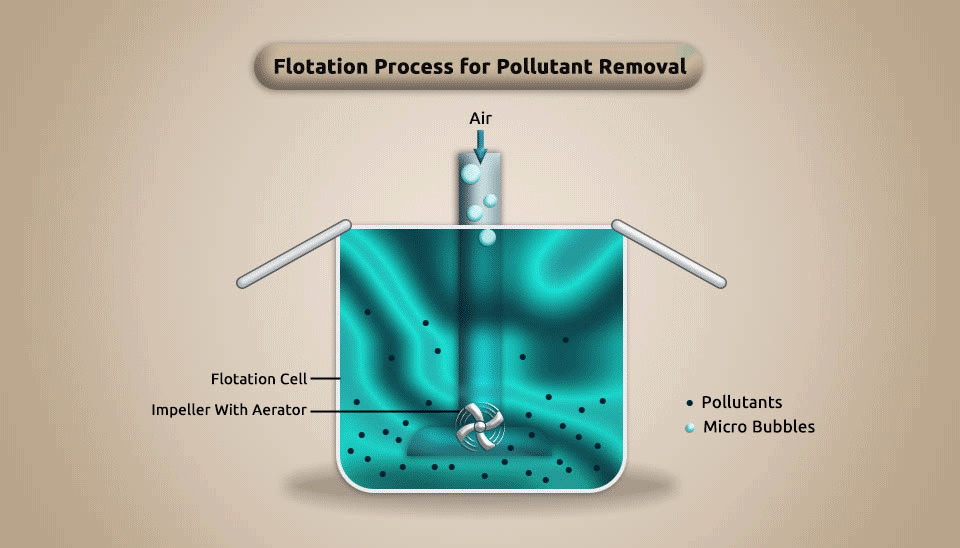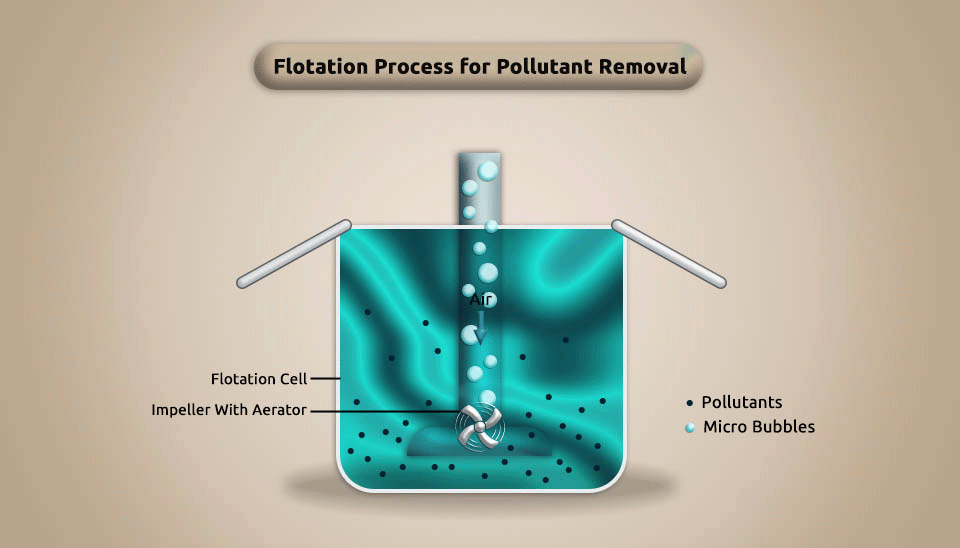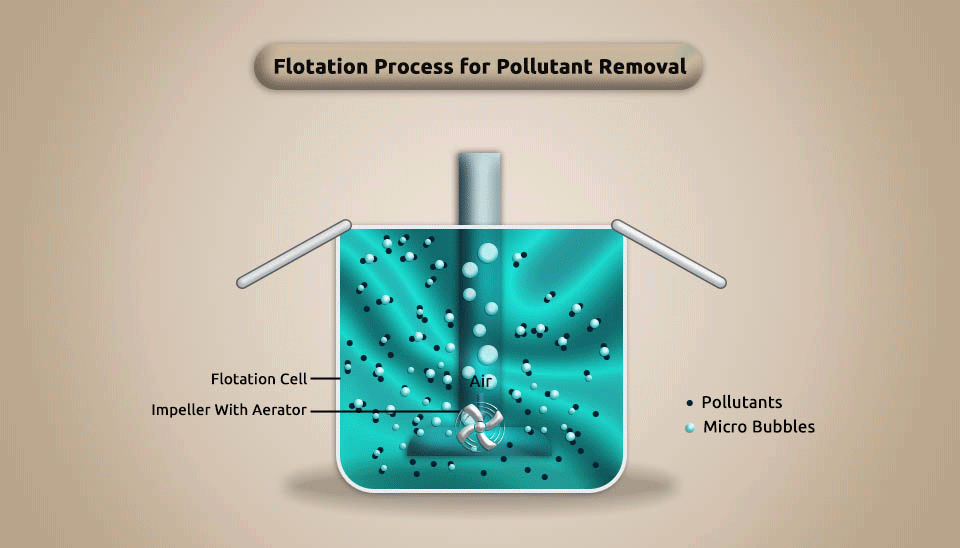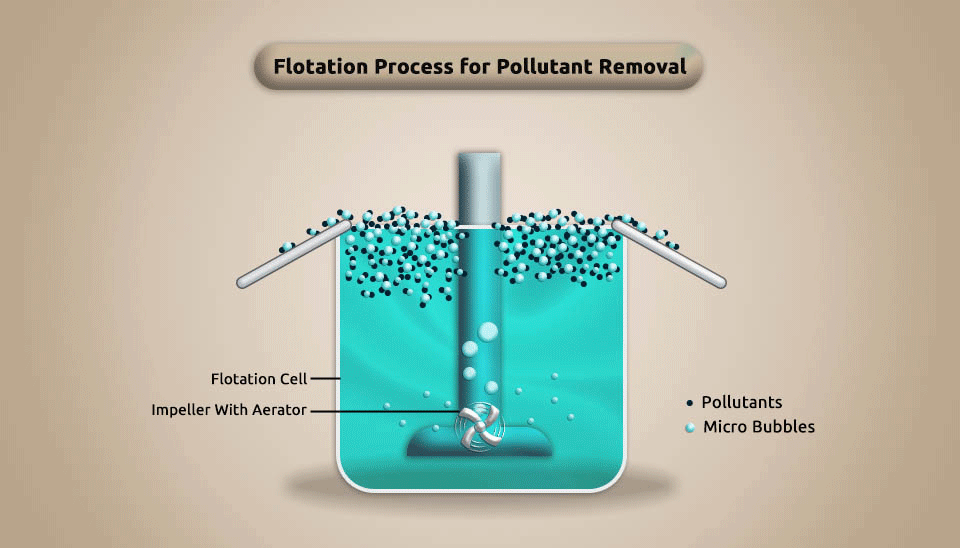
2.1.2. Flotation
To achieve the goal of separating and removing pollutants, the flotation process accesses a large number of microbubbles in the water to make it adhere to the concentration of similar pollutants in pharmaceutical wastewater. This procedure causes the pollutants to float to the water surface. Depending on how microbubbles are made, the flotation process can be split into three groups: electro-flotation, induced air flotation, and dissolved air flotation.
The flotation technique is frequently utilized to separate microscopic particles whose density is similar to or lower than that of water. It has the advantages of being energy-efficient, affordable, and simple to use and operate. However, it is considered an expensive method, because of its high capital and maintenance cost (Suarez et al., 2009). Fig. 3 depicts the flotation process’s schematic.
2.1.3. Membrane Separation
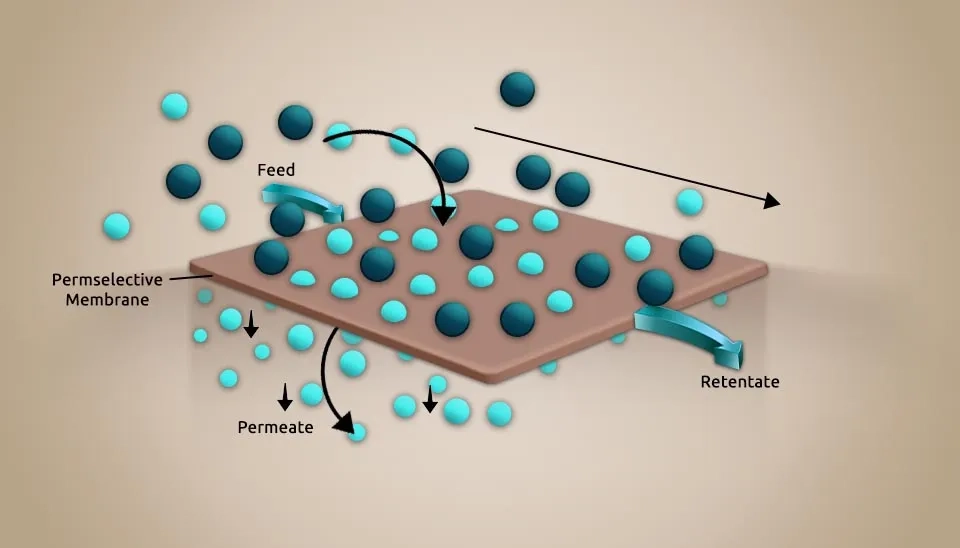
Diffusion-dialysis, electrodialysis, Reverse Osmosis (RO), and UltraFiltration (UF) are examples of membrane separation processes that utilize external energy or chemical potential difference as a driving force to eliminate pollutants from pharmaceutical wastewater (Fig. 4). Membrane separation can handle pharmaceutical wastewater that is challenging to treat using conventional techniques, and the treatment effect is not significantly impacted by changes in water quality. The advantages of this technology include a straightforward procedure, convenient operation, no need for large spaces, and no change in the sewage's composition. However, the membrane module's limited lifetime, maintenance cost, and vulnerability to contamination can be challenging (Martinez et al., 2013).
2.1.4. Adsorption
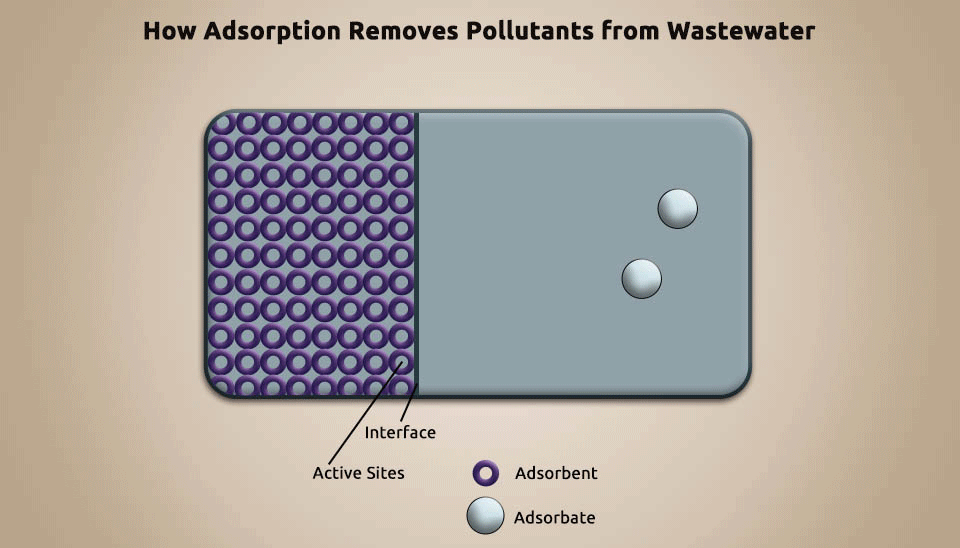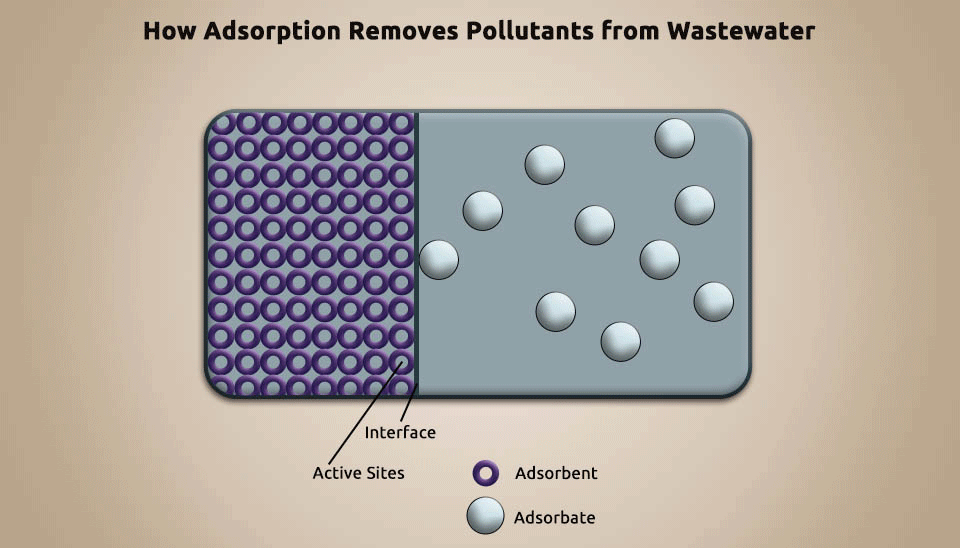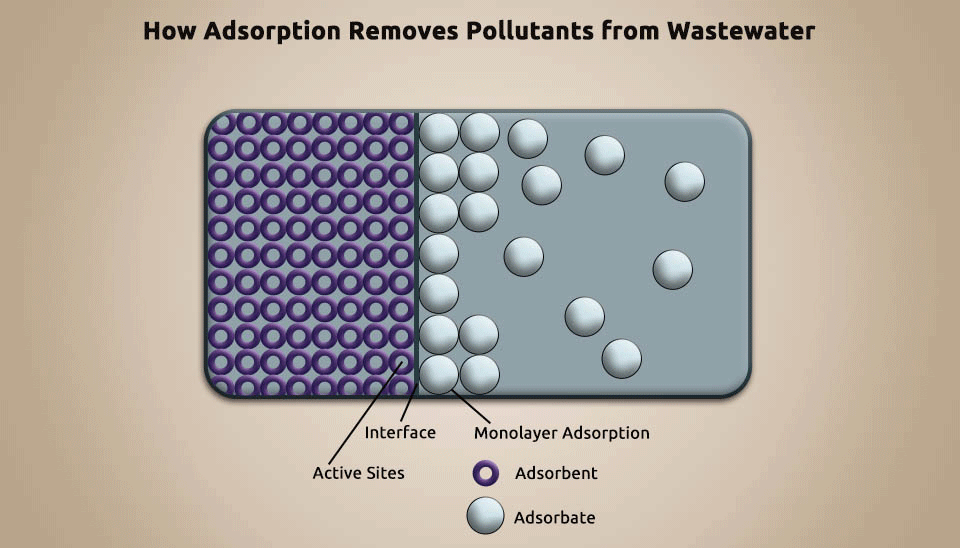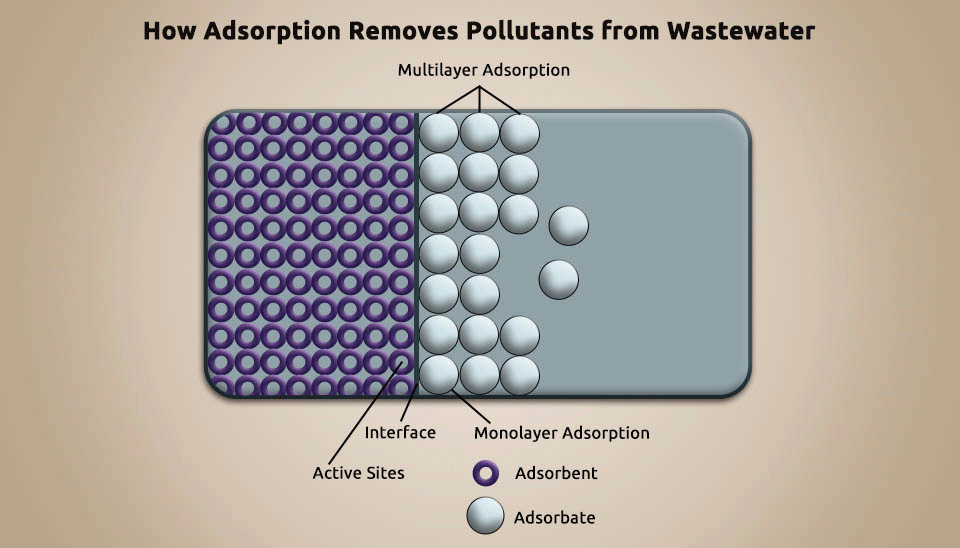
Adsorption is a technique utilized to eliminate pollutants from pharmaceutical wastewater by using porous particles, such as activated carbon, silica gel, and artificial pumice, to adsorb the contaminants (Fig. 5). Adsorption can improve biochemicals, recover useful chemicals, and diminish the concentration of refractory organic matter in pharmaceutical wastewater. It is a cost-efficient, tremendous method for organic contaminants removal ; nevertheless, it requires constantly regenerating the adsorbent and that makes it not affordable in numerous instances. (Putra et al., 2013).
2.1.5. Electrolysis
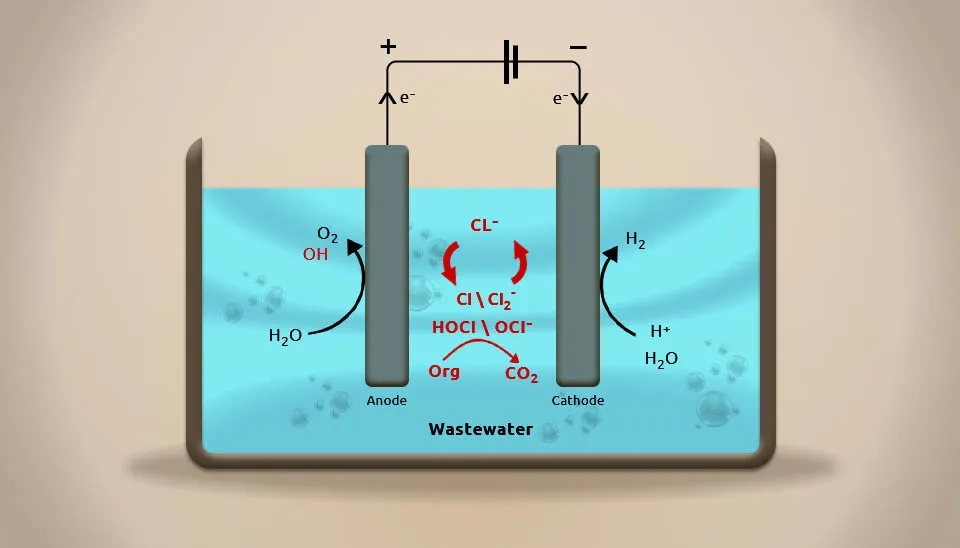
The electric current application results in various chemical processes that change the structure and behavior of organic contaminants in pharmaceutical wastewater. The hydrogen and oxygen created at the electrode's two levels can be used to oxidize and eliminate pollutants in pharmaceutical wastewater (Fig. 6). Electrolysis works in a number of ways, with electrochemistry being one of the most important. Flotation and flocculation are also important for getting rid of contaminants in pharmaceutical wastewater, but they do so in different ways. The advantages of the electrolysis process are easy operation and control, consistent effluent quality, a strong decolorization impact, and low area coverage. However, due to the significant energy consumption, processing costs are high (Ji et al., 2017).
2.2. Chemical Processes
Pharmaceutical wastewater undergoes treatment through chemical processes. Among the different types of these processes, three are the most common: Fenton, Ozonation, and PhotocatalyticOxidation.
2.2.1. Fenton
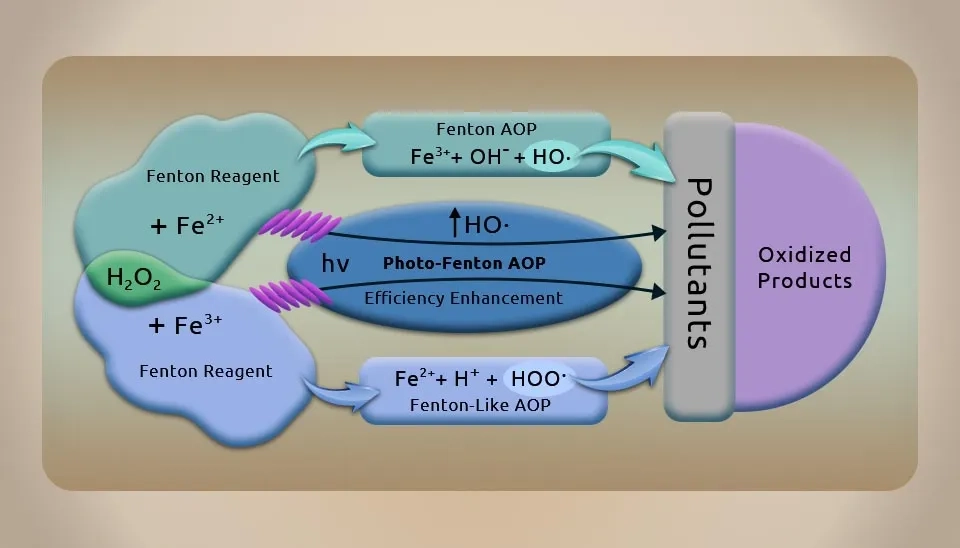
Hydrogen peroxide and ferrous salt are combined to form the Fenton reagent. To oxidize the organic molecules in pharmaceutical wastewater, ferrous salt can accelerate the formation of hydroxyl radicals (•OH) from hydrogen peroxide, which features a very high oxidation potential. The Fenton process (Fig. 7) is appropriate for treating pharmaceutical wastewater with high concentrations of contaminants that are hard to break and are toxic, or as a pretreatment before biological treatment, due to the non-selectivity and severe oxidizing ability of •OH (E0=2.8eV), which may decompose a variety of pollutants. Fenton oxidation has features like straightforward operation, mild reaction conditions, and inexpensive equipment that make it preferable. However, the effluent contains a large number of iron ions and needs additional treatment (Segura et al., 2014).Fig. 7. The schematic of the Fenton Process
2.2.2. Ozonation
Ozone has the greatest redox potential of all regularly used chemical oxidants (with a redox potential of 2.07V) and is the strongest disinfectant and sterilant. There are two different types of oxidation: direct reaction and indirect reaction. Direct reaction employs the ozone's powerful oxidation to oxidize pollutants right away. An indirect reaction occurs when ozone breaks down in water to produce highly reactive •OH, which oxidizes pollutants in pharmaceutical wastewater. The biodegradability of pharmaceutical wastewater is improved by ozone, which performs the functions of open-loop and chain scission to transform macromolecular organisms into biodegradable small-molecule organic compounds. The advanced treatment of biological treatment effluent, which has a positive treatment impact on organic pollutants of high stability and refractory in pharmaceutical wastewater, or the pretreatment of high concentration, rarely biodegradable pharmaceuticalwastewater, is therefore particularly suitable for ozonation. The advantages of this method include its quickness, effectiveness, lack of secondary contaminants, and minimal sludge leftover. This method is highly active and toxicity-free. However, costly operation and maintenance are drawbacks of this method. Fig. 8 shows the schematic of the ozonation process (Liu et al., 2014).
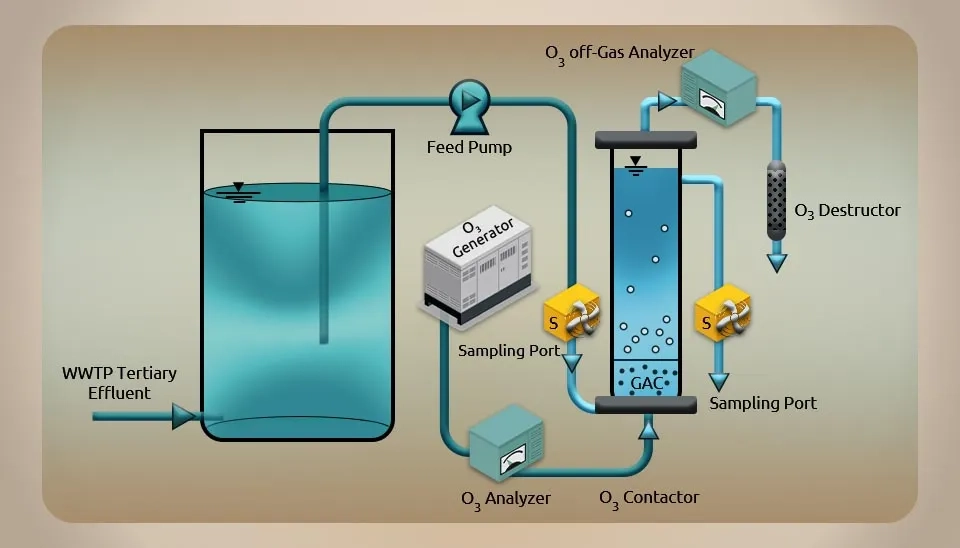
2.2.3. Photocatalytic Oxidation
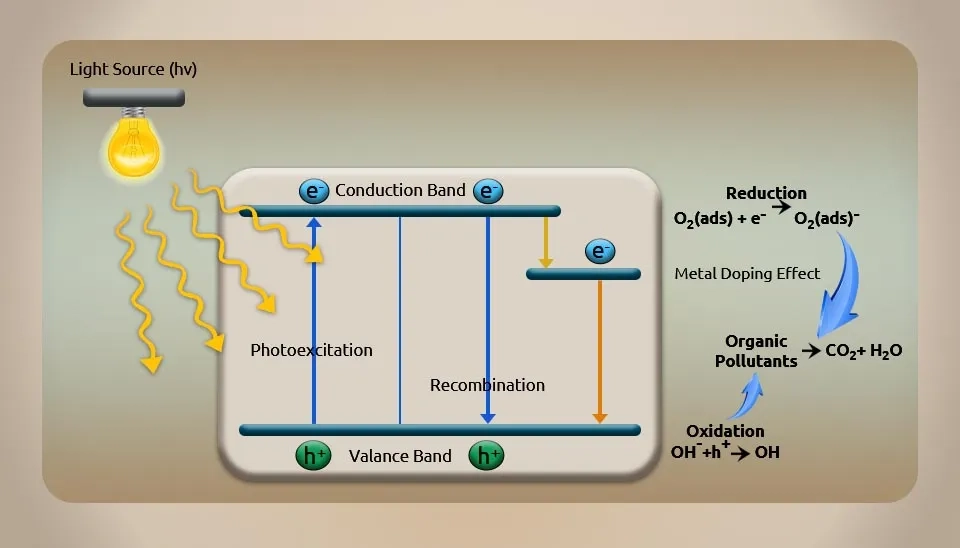
The photocatalytic oxidation process (Fig. 9) uses n-type semiconductors like TiO₂ SrO₂ WO₃SnO₂ and others as photochemical degradation catalysts. Electron-hole pairs (h⁺-e⁻), which are produced when these catalysts are exposed to ultraviolet light, will move to the semiconductor surface and produce •OH, which can oxidize contaminants in pharmaceutical wastewater (Kanakaraju et al., 2014). With its powerful oxidizing ability, lack of secondary pollution, and mild reaction conditions, the photocatalytic oxidation process turns refractory organic pharmaceutical wastewater into an effluent with less harmful effects on the environment. There are some benefits to this method, such as having no secondary contaminants and a significant capacity for oxidizing, although the oxidative byproducts and high operational expenses are the drawbacks.
2.3. Biological Processes
Pharmaceutical wastewater shouldn't, in theory, be handled biologically after secondary treatment due to its poor biodegradability. We cannot, however, disregard the benefits of biological treatment, such as its low cost and consistent results. We can apply it as a pretreatment in more sophisticated care.
2.3.1. Sequencing Batch Reactor (SBR)
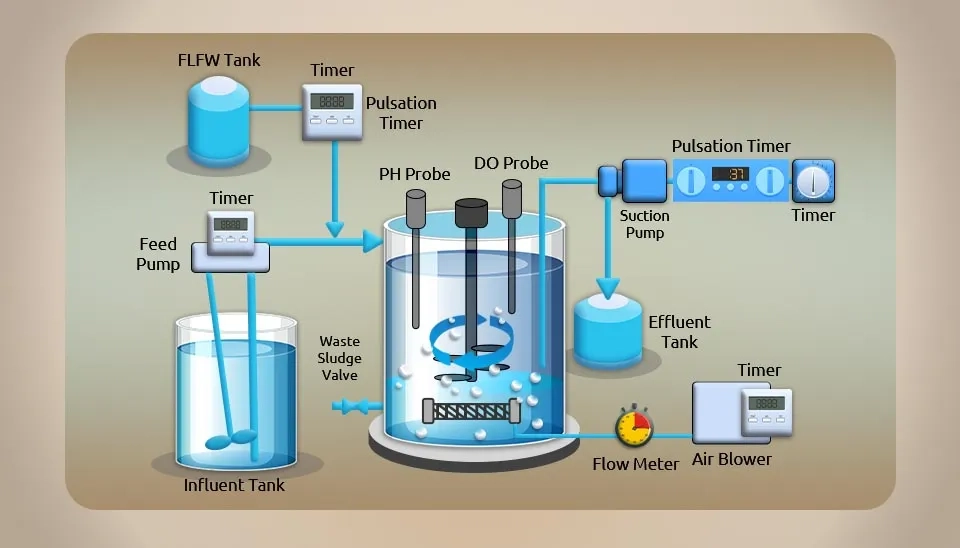
The flow pattern of a Sequencing Batch Reactor (SBR), which is a "water injection - reaction - drainage" type of reactor, is quite mixed. It is a type of intermittent aeration-based technology for treating activated sludge wastewater that includes the five essential stages of injection, reaction, precipitation, drainage, and idleness (the schematic diagram of an SBR process is depicted in fig. 10). Since all processes run concurrently in a reactor with an aeration stirring device, there is no need for a separate sedimentation tank (Shi et al., 2014). The method is suitable for treating pharmaceutical wastewater with significant changes in water quality and quantity because of its benefits in simplicity of process, flexibility of operation, strong compliance with impact resistance, and steady effluent quality. However, the SBR process can be considered a hard method to maintain and manage and requires a lot of time to complete the sludge settling and separation; thus, it's important to maintain a high sludge concentration while treating pharmaceutical wastewater with a high concentration to avoid high viscosity bulking.
2.3.2. Upflow Anaerobic Sludge Blanket (UASB)
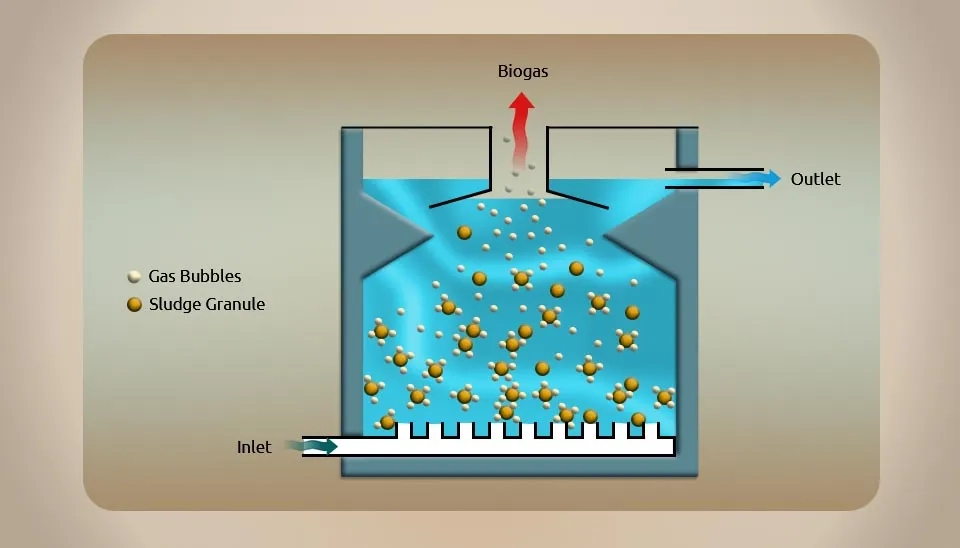
The reactor of the upflow anaerobic sludge blanket (UASB) process, an anaerobic biological treatment method, is primarily made up of the three-phase separator, gas chamber, reaction zone, and treated water discharge system (the schematic diagram of a UASB process is shown in Fig. 11). When pharmaceutical wastewater flows through the reactor from bottom to top, the highly active sludge at the bottom of the reactor transforms most of the organic pollutants into CH₄ and CO₂. A sludge suspension layer forms above the sludge layer as a result of the digestion gas stirring up and the bubbles sticking to the sludge (Alvarino et al., 2014). This layer requires a three-phase separator to accomplish the separation of the gas, liquid, and solid phases. The advantages of the UASB reactor include its small size, high processing capacity, lack of mechanical stirring, effective treatment, and low capital costs. However, it’s difficult in microbial domestication and challenging to manage. The intermittent pulse influent must compensate for the lack of interaction between the bacterial cells and the matrix that results from insufficient gas generation during the initial stage of start-up.
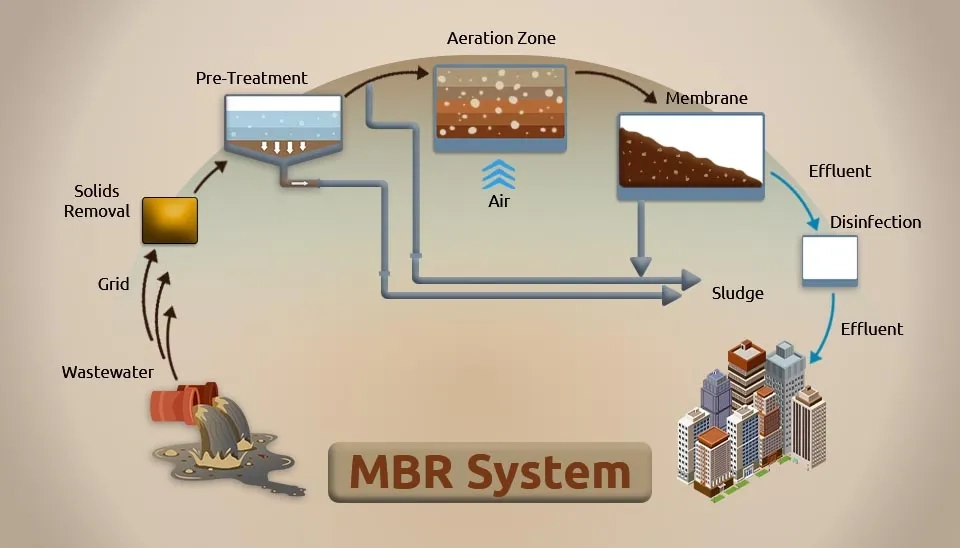
2.3.3. Membrane Bioreactor (MBR)
The membrane bioreactor(MBR) process combines membrane technology with biological wastewater treatment technology and separates sludge's solid and liquid components using an ultrafiltration membrane instead of a secondary sedimentation tank (Fig. 12). The MBR process, a sewage treatment technology that integrates concentration and separation, can intercept activated sludge and macromolecular organic pollutants through membrane separation devices to resolve the issue of sludge expansion in the conventional activated sludge process. Additionally, it has flexible controls for hydraulic retention time and sludge residence time. It also has automatic control, increases the concentration of activated sludge in the aeration tank, and speeds up biological degradation. This increases the effectiveness of pollutant removal and often allows for direct recycling of the effluent (Kaya et al., 2016). Strong microbial biomass interception, excellent operational stability, and good effluent quality are all benefits of membrane bioreactors. However, there are still several drawbacks, including high cost, simple module contamination, and short membrane service life.
3. Conclusion
The pharmaceutical industry is one of the largest and most famous in the world, and the treatment of pharmaceutical wastewater is a global concern. At the same time, the necessity for a sustainable and efficient pharmaceutical wastewater treatment method is highlighted by the growing severity of the contamination produced by pharmaceutical wastewater. Pharmaceutical wastewater has some characteristics, such as poor biodegradability and high concentration, due to the complexity of pharmaceutical processes. Because of these traits, pharmaceutical wastewater requires highly advanced treatment. There are many different types of advanced treatment, and each one hasits advantages and disadvantages. The quality of pharmaceutical wastewater effluent can be effectively improved via the precise application of diverse technologies and methods.
