
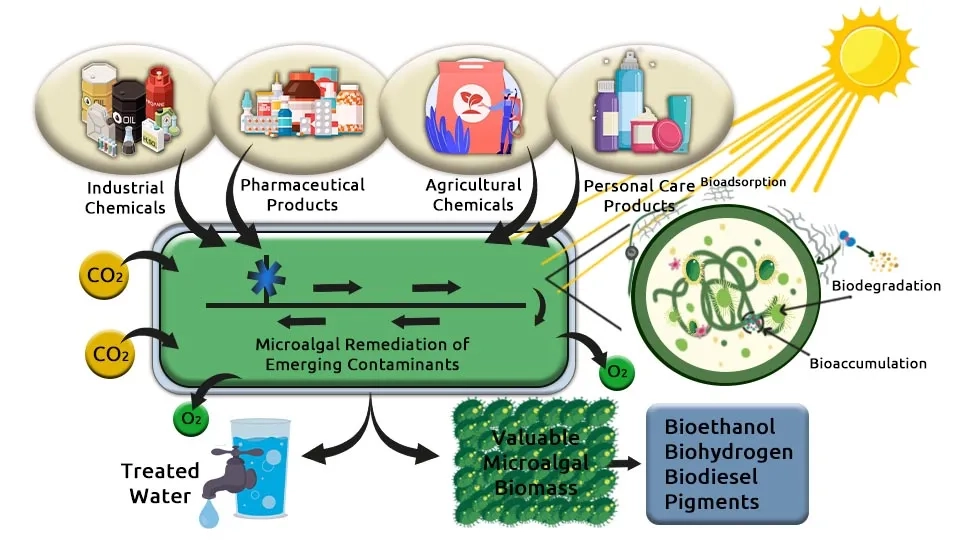
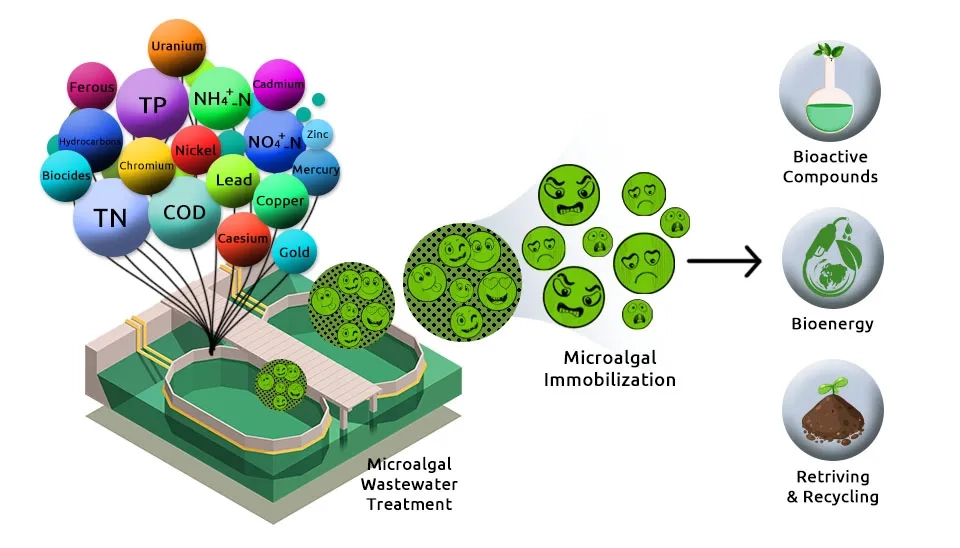
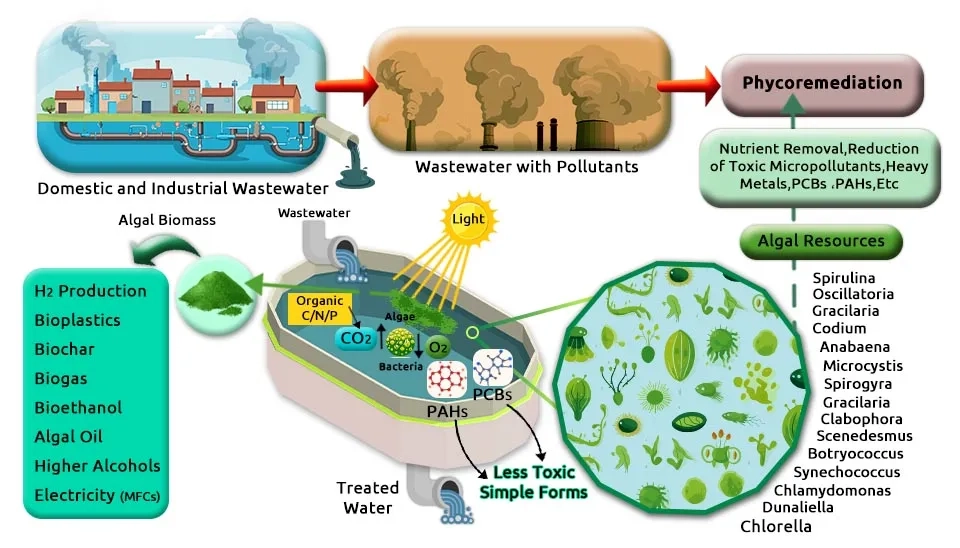
How can we clean the world’s wastewater without poisoning our environment in the process? Traditional treatment relies on a process so energy-intensive it accounts for an estimated 15 to 20 percent of municipal energy consumption globally, while still failing to remove up to 90 percent of certain Emerging Contaminants (ECs) like pharmaceuticals and pesticides. This approach is costly, chemically-reliant, and unsustainable. This article introduces a paradigm shift: Biological Wastewater Treatment—microalgae bioremediation. This contrasts sharply with traditional Primary Wastewater Treatment methods. By harnessing specialized microalgae as living, green scrubbers, we can naturally filter and neutralize pollutants while simultaneously sequestering carbon. We present a groundbreaking solution—optimized microalgae consortia—powerful combinations engineered for the simultaneous and complete cleanup of nine critical pollutants, paving the way for truly green water management.
These processes can be very energy-intensive and expensive, consuming between 80 and 240 kWh per million gallons and at a cost between $0.70 and $2.90 per million gallons treated, respectively (Muga and Mihelcic, 2008). The release of newly discovered pollutants into the environment is one of the main issues of our day. These not only have implications for ecosystems but also pose significant threats to human health. One of the main causes of water pollution is agriculture alone. Tens of millions of tons of fertilizers, pesticides, insecticides, and herbicides—approximately 140 million tons used by farmers annually (Kurade et al., 2016). Environmental concerns necessitate urgent concerns about sustainability in relation to pollution prevention and water treatment.
For a deeper understanding of the synergistic relationship and sustainable outcomes, read more about the Microalgae-Bacteria Consortium.
Table 1. Which pollutants can different microalgae effectively remove? The table below presents 59 microalgae, each identified for its ability to remove specific types of pollutants based on 185 studies.
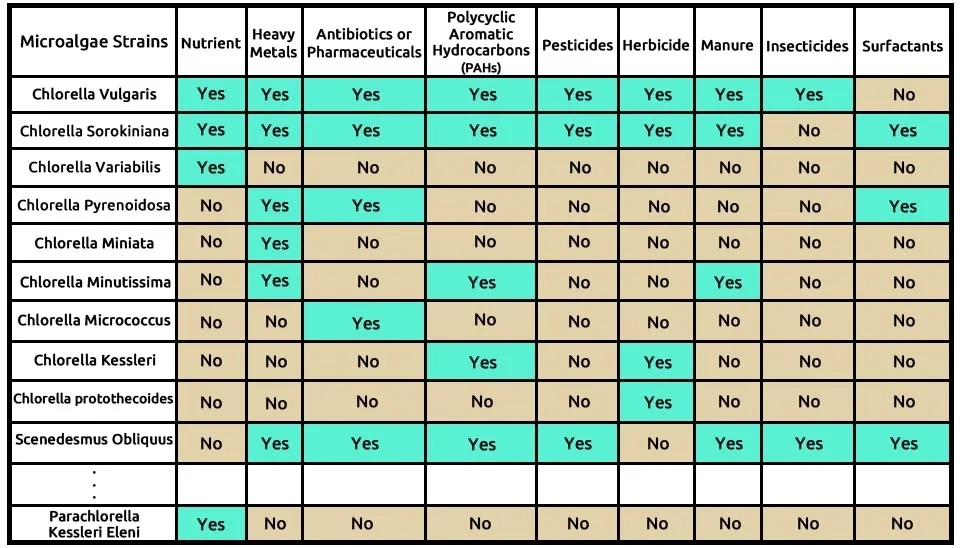
Click this link to see the full specification table
Microalgaee Consortia Wastewater Pollution Removal
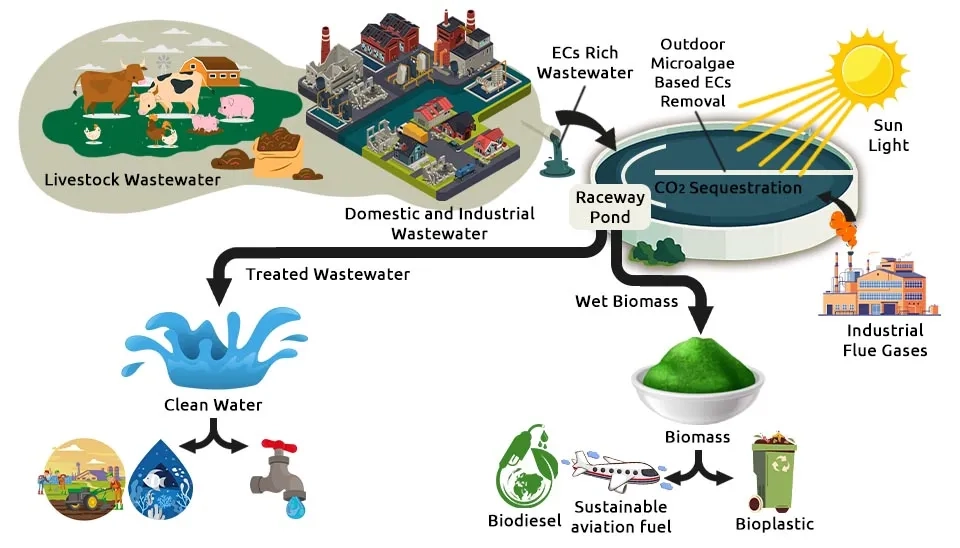
Surfactants are considered another one of the pollutants in the aquatic and terrestrial environment. Twenty years ago, over 4.2 Million Tons (MTs) of detergent products and 1.2 MTs of softener products were used annually in Western Europe (Arora et al., 2022). How should these pollutants in water sources be addressed? Creating wastewater systems that employ appropriate technology and are cost-effective is crucial for ensuring the efficient operation of wastewater treatment plants across many countries. Effective steps, often including the use of Coagulants, must be taken to ensure efficient operation. One effective technology is the use of microalgae systems for effluent reclamation, which simultaneously provides oxygen production via photosynthesis and nutrient recycling, including nitrogen (N), phosphorus (P), and other compounds assimilated into the microalgae biomass (González and Marciniak, 2008).
This article introduces combinations of microalgae strains capable of removing nine specific pollutants, including 1) nutrients, 2) heavy metals, 3) antibiotics or pharmaceuticals, 4) Polycyclic Aromatic Hydrocarbons (PAHs), 5) pesticides, 6) herbicides, 7) manure, 8) insecticides, and 9) surfactants. The author has suggested these combinations, and you can use the link below to get your preferred combination.These processes are critical components of modern Industrial Wastewater Treatment Methods.
1. CRT Microalgae Combination
The research suggests a combination of three microalgae—Chlorella vulgaris (C), Rhoicosphenia abbreviata (R), and Tetraselmis suecica (T)—which are capable of removing pollutants, as shown in Table 2. Chlorella vulgaris can remove many contaminants, such as nutrients from municipal wastewater, heavy metals, antibiotics or pharmaceuticals, PAHs, pesticides, manure, and insecticides. Rhoicosphenia abbreviata is effective in breaking down herbicides, while Tetraselmis suecica specifically removes surfactants. The high concentration of surfactants and other contaminants often found in Greywater makes this consortium approach highly valuable for reclamation projects. The combination of microalgae, including Chlorella vulgaris, Rhoicosphenia abbreviata, and Tetraselmis suecica, in the removal of nine pollutants from emerging contaminants (ECs) is presented in Table 2.
Table. 2. ECs removal by microalgae strains
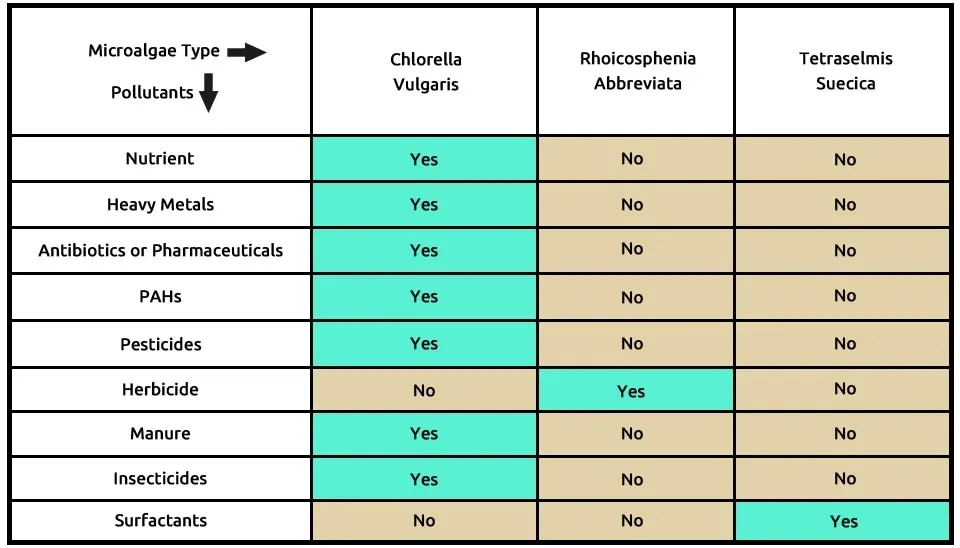
Click this link to see the full specification table
Table 2. ECs removal by microalgae strains
Chlorella vulgaris can grow in various environments, including landfill leachate, dairy-derived liquid digestate, mine tailings water, agricultural runoff, groundwater, fresh manure, and several types of wastewater (piggery, starch, municipal, and synthetic) (Ferrando and Matamoros, 2020; Castellanos-Estupiñan et al., 2022; Gonçalves et al., 2016; Huo et al., 2020; Okurowska et al., 2021). These microalgae degrade organic pollutants, including pharmaceuticals, insecticides, and herbicides, through the action of specific enzymes. This approach is sometimes integrated with advanced processes like Membrane Bioreactor Technology. The synergy with Bacterial Consortia further enhances the removal efficiency of Emerging Contaminants (ECs).Chlorella vulgaris absorbs and accumulates various pollutants due to its extensive surface area, diverse functional groups, and ability to perform photosynthesis (Hsiao et al., 2021). Tao et al. (2017) developed a unique Algal Biofilm Airlift Photobioreactor (ABA-PBR) for the attached growth of Chlorella vulgaris on sewage. Chlorella vulgaris improved nutrient removal rates of 1.0 and 0.2 mg L−1 d−1 for N and P, respectively, at reactor temperatures between 25 and 30°C with a maximum light intensity of 120.8 μmol m−2 s−1 in 37 days.
Urrutia et al. (2019) investigated Cu (64.7%) and Mo (99.9%) removal from Mine Tailings Water (MTW) by Chlorella vulgaris at a temperature of 20 ± 3°C using cool white fluorescent lamps at 60 μmol m−2 s−1 in 196 h. Xiong et al. (2017) achieved the removal of Enrofloxacin (18–26%) antibiotics using microalgal species, including Chlorella vulgaris, under a light intensity of 45–50 μmol photons m−2 s−1, a 16:8 light/dark cycle, a temperature of 27°C, and a shaking speed of 150 rpm by day 11. Tomar and Jajoo. (2021) investigated the bioremediation of fluoranthene (FLT), a polycyclic aromatic hydrocarbon (PAH), by Chlorella vulgaris, achieving a 90–94% removal under a light intensity of 100 µmol m−2 s−1, at 25°C, and with a diurnal cycle of 16 hours of light and 8 hours of darkness over 7 days. Hultberg et al. (2015) studied a wide range of different pesticides (50–80%) in water using C. vulgaris at 20°C and illumination of 100 µmol m-2 s-1 for 4 days. Franchino et al. (2013) investigated the removal of dairy manure (96%) using microalgal species, including C. vulgaris, at a temperature of 25 ± 2°C and continuous artificial illumination of 200 µmol m-2 s-1 over 21 days. Kurade et al. (2016) demonstrated the effective removal of diazinon (94%) from the aqueous phase by freshwater Chlorella vulgaris, cultivated at 150 rpm, 27°C, and a light intensity of 45–50 μmol photons m−2 s−1 after 12 days of cultivation.
Ricart et al. (2009) employed a recirculating channel system utilizing the autotrophic activity of microalgae community composition, including Rhoicosphenia abbreviata, Surirella brebissonii and Cyclotella meneghiniana to achieve 96% removal of the herbicide diuron after 8 days.
Biofilm samples were grown under an irradiance level of 120 μE m−2 s−1 with a 12:12 h light-dark cycle and a flow rate of 1.5 L min−1. The water temperature was approximately 17°C, and the mean conductivity was 2.02 mS cm−1 in both of treated and control channels. Ulloa et al. (2012) initially evaluated the lytic effect of two common surfactant families, Triton and Tween, on the cell walls of the microalga Tetraselmis suecica after 2 days of contact. T. suecica was cultured in seawater with 3.5% salinity enriched with nutrients and a light intensity of 596 μmol quanta m−2 s−1 at 20°C and pH of 7–8, with a 12-hour light and 12-hour dark cycle.

Kassim and Bhattacharya. (2016) investigated the pretreated biomass and enzymatic saccharification of Tetraselmis suecica and Chlorella sp. The biomass resulting from microalgae wastewater treatment must be managed through specialized Sludge Treatment Processes before reuse or disposal. The maximum reducing sugar concentration was obtained from T. suecica (81 mg/g dried biomass) when the biomass was pretreated with 2% (w/v) potassium hydroxide (KOH) at 120°C for 120 minutes. Niccolai et al. (2019) investigated the biochemical composition, the fatty acid profile, and the vitro digestibility of two Chlorella vulgaris and Tetraselmis suecica microalgal biomasses. The three types of cyanobacteria and Chlorella species contained a high protein content (50–65%) and low fat content (5–20%). T. suecica, P. purpureum, and P. tricornutum grow in a nutrient-rich medium, containing 14–17% nutrients. Chlorella vulgaris, Rhoicosphenia abbreviata, and Tetraselmis suecica can coexist and grow together under specific conditions. However, it is important to note that different microalgae species have varying growth requirements, including light intensity, temperature, pH, and nutrient availability. Therefore, it is necessary to optimize the culture conditions to support the simultaneous growth of all three species. Additionally, competition for nutrients and space may arise between the different species, affecting their growth rates. Effective management and monitoring of the culture system would be required to achieve successful co-cultivation of these microalgae species.
2. CCA Microalgae Combination
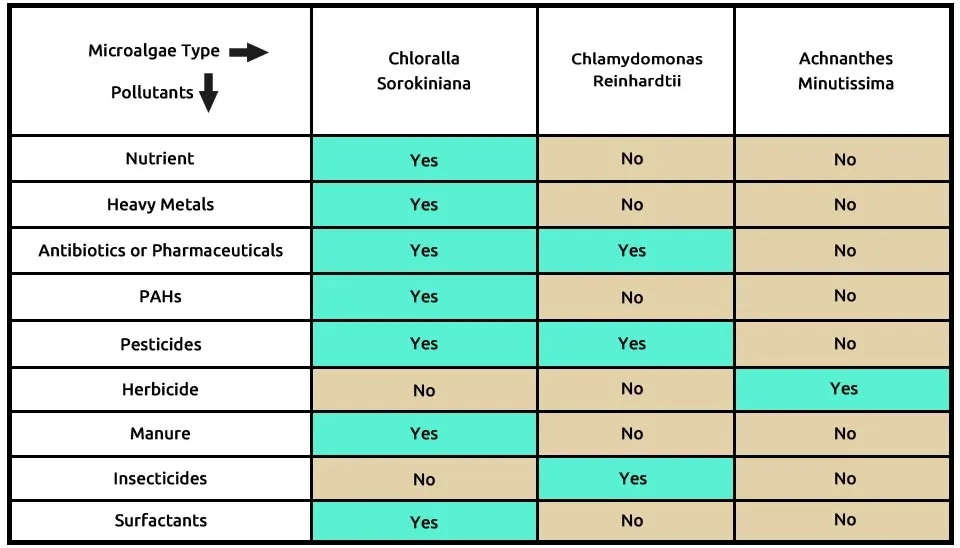
We suggest the second combination of three microalgae—Chlorella sorokiniana (C), Chlamydomonas reinhardtii (C), and Achnanthes minutissima (A)—which can remove pollutants, as shown in Table 3. Chlorella sorokiniana, similar to Chlorella vulgaris, can remove a wide range of contaminants, such as nutrients from municipal wastewater, heavy metals, antibiotics or pharmaceuticals, PAHs, pesticides, manure, and surfactants. Research shows that Chlamydomonas reinhardtii removes contaminants such as antibiotics or pharmaceuticals, pesticides, and insecticides, while Tetraselmis suecica removes herbicides only. The composition of microalgae, including Chlorella sorokiniana, Chlamydomonas reinhardtii, and Achnanthes minutissima in the removal of 9 pollutants from Emerging Contaminants (ECs) is presented in Table 3..
Table 3. ECs removal by microalgae strains
Click this link to see the full specification table
Table 3. ECs removal by microalgae strains
Eladel et al. (2018) grew Chlorella sorokiniana in Bold’s Basal Medium (BBM) as a control, municipal Wastewater (WW), and wastewater enriched with BBM elements (WW+).
During a 10-day cultivation period in municipal wastewater (WW), Chlorella sorokiniana achieved removal efficiencies of 74.2% for NO3–N, 83.3% for NH3–N, and 78.0% for total phosphorus (TP) at a temperature of 25 ± 1°C and a light intensity of 100 µmol photons m−2 s−1.This is a common application for treating Domestic Wastewater Sources. Petrovic and Simonic. (2016) investigated the capacity of Chlorella sorokiniana for removing Cu2+ (97.10%), Ni2+ (50.94%), and Cd2+ (64.61%) ions from drinking water solutions. Chlorella sorokiniana was cultivated in Bold’s Basal growth media under room conditions after 10 days. Wilt et al. (2015) studied micropollutant removal in an algal treatment system fed with source-separated wastewater streams using Chlorella sorokiniana. The removal for six spiked pharmaceuticals—diclofenac, ibuprofen, paracetamol, metoprolol, carbamazepine, and trimethoprim—ranged from 30% to 100%. C. sorokiniana was cultivated at 35°C in 250 ml shake flasks with 3% CO2 (v/v) and continuous irradiation averaging of 80 µmol m−2 s−1.
Nanda et al. (2019) evaluated the effect of malathion on the growth and biochemical parameters of Chlorella sorokiniana. The C. sorokiniana culture degraded 90% of the malathion pesticide within 14 days. Microalgae were cultivated under a photoperiod of 18 hours light and 6 hours dark, with a light intensity of 200 µmol photons m−2 s−1 and a temperature of 25°C. González et al. (2008) conducted batch experiments using Chlorella sorokiniana for swine manure removal, achieving 75% removal efficiency after 500 hours. Chlorella sorokiniana and Scenedesmus obliquus were incubated at 30°C under continuous illumination at 4,500 lx. Janoska et al. (2018) demonstrated the potential for a novel liquid foam-bed photobioreactor for microalgae growth in the presence of different surfactants (non-ionic, cationic, and anionic). C. sorokiniana was cultivated on 3 times concentrated M8a media at 37 °C, under a light intensity of 54 µmol m-2 s-1, 120 rpm, and 4% CO2.
Jin et al. (2012) analyzed the bioaccumulation and degradation of herbicide pyrometry in Chlamydomonas reinhardtii. C. reinhardtii removed prometryne pesticide at concentrations 2.5–12.5 µg L-1 for 4 days. The microalgae was grown in 150 mL Erlenmeyer flasks containing 50 mL of axenic BG-11 medium (pH 7.1) under conditions of 14-hour photoperiod (day/night), with a light intensity of 80 µmol m-2 s-1 between 20°C and 20°C. Yılmaz and Taş. (2021) used Chlamydomonas reinhardtii to remove 98.2% of the insecticide zeta-cypermethrin within 96 hours. C. reinhardtii was cultivated under a 16:8-hour light/dark cycle at a temperature of 24°C. Martini et al. (2021) investigated the ability of maize roots to stimulate the growth of Chlamydomonas reinhardtii and Chlorella sorokiniana for microalgae cultivation at an industrial scale. The findings show that both C. reinhardtii and C. sorokiniana enhanced maize root growth compared to the untreated control. Hom-Diaz et al. (2020) studied the ability of four selected microalgae species, including Chlamydomonas reinhardtii, Chlorella sorokiniana, Dunaliella tertiolecta, and Pseudokirchneriella subcapitata, to remove nine antibiotics and the antidepressant venlafaxine under different experimental conditions. Research has shown that two species of microalgae, chlorella sorokiniana and Chlamydomonas reinhardtii, have been successfully co-cultivated and are compatible. The compatibility of the Achnanthes minutissima microalgae under optimum cultivation conditions should be further investigated.
3. SCG Microalgae Combination
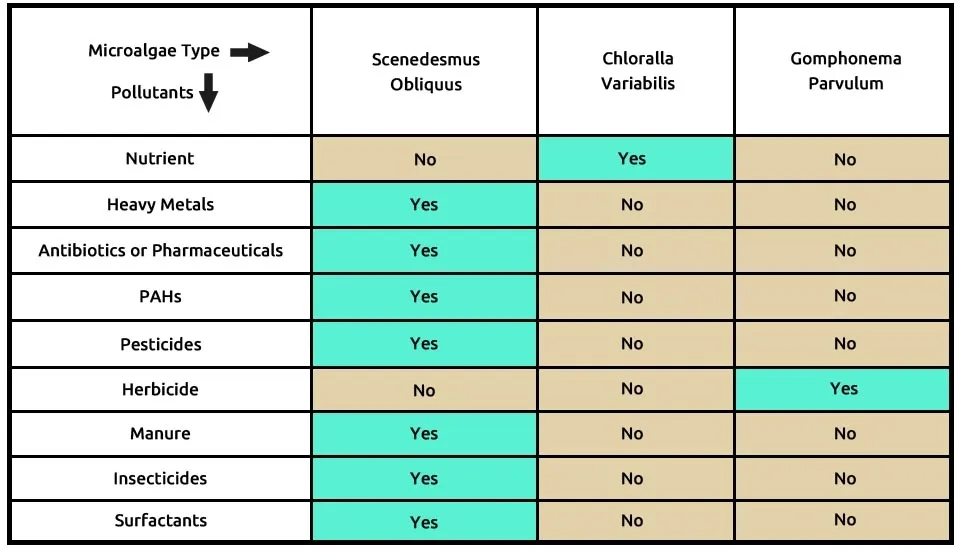
The third suggested combination is the three microalgae, including S (Scenedesmus obliquus), C (Chlorella variabilis), and G (Gomphonema parvulum), which can break down contaminants, as shown in Table 4. Scenedesmus obliquus, similar to Chlorella sorokiniana and Chlorella vulgaris, can remove several contaminants, including heavy metals, antibiotics or pharmaceuticals, PAHs, pesticides, insecticides, manure, and surfactants. Due to their resilience, three Scenedesmus obliquus species, like Chlorella sorokiniana and Chlorella vulgaris microalgae, exhibit higher pollution removal efficiency, suggesting they belong in the same group. Chlorella variabilis effectively removes nutrients from municipal wastewater, while Gomphonema parvulum specifically targets herbicides. The composition of microalgae, including Scenedesmus obliquus, Chlorella variabilis, and Gomphonema parvulum, in the removal of nine pollutants from ECs is presented in Table 4.
Table 4. ECs removal by microalgae strains
Click this link to see the full specification table
Table 4. ECs removal by microalgae strainsTable 4. ECs removal by microalgae strains
Rinanti et al. (2018) investigated the biosorption of copper (40–100%) from electroplating wastewater by biosorbent using Scenedesmus obliquus in the artificial medium of Phovasoli Haemotococcus Medium (PHM) at a pH of 6, a temperature of 28°C ± 2, and an ambient light intensity of 5000 lux over 240 minutes. Xiong et al. (2017) removed 18-26% of the Enrofloxacin antibiotic, a fluoroquinolone antibiotic, using five individual microalgae species, including Scenedesmus obliquus, after 11 days of cultivation. Scenedesmus obliquus grows under a light intensity of 45–50 µmol m-2 s-1, 16:8 light/dark cycle, at a temperature of 27°C, and with a shaking speed of 150 rpm. Van Do et al. (2020) proposed an economic strategy for the feasible commercialization of the microalgae industry. They studied the growth and pollutant removal efficiency of Chlorella variabilis using domestic wastewater as the sole nutrient source in raceway ponds. C. variabilis achieved nutrient removal efficiencies in the reactor systems, with the Chemical Oxygen Demand (COD) reduced by 85.1–96.8%, total nitrogen by 64.7–90.7%, and total phosphorus by 99.7%–100%.
Wood et al. (2016) investigated the removal of herbicides (48%) such as atrazine, simazine, hexazinone, tebuthiuron, and diuron; as well as 2-Methyl-4-Chlorophenoxyacetic Acid (MCPA), 2,4-Dichlorophenoxyacetic acid (2,4-D), and 5-Enolpyruvylshikimate 3-Phosphate (EPSP) by Gomphonema parvulum at 48 hours. The laboratory was maintained at a temperature of 24 ± 2°C with a light intensity of 20 μmol m−2 s−1 under a 12-hour light/dark cycle.
Loganathan et al. (2020) assessed the ability of the microalgal consortia composed of Chlorella variabilis and Scenedesmus obliquus to treat and valorize diluted synthetic dairy wastewater under controlled laboratory conditions. Under optimized experimental conditions, the microalgae consortia removed 70.19% of phosphate, 86.22% of ammoniacal nitrogen, and 54.72% of COD. Several studies have focused on the combination of Chlorella variabilis and Scenedesmus obliquus microalgae, demonstrating their potential for pollutant removal. However, Gomphonema parvulum cultivation had not been considered in combination with these microalgae. Thus, further studies are required in establishing conditions for cocultivation between Scenedesmus obliquus, Chlorella variabilis, and Gomphonema parvulum that ensure compatibility and maximize effectiveness in contaminant removal. Future advancements, including the application of Nanotechnology in Water Treatment, will continue to expand the feasibility and efficiency of microalgae systems.
4. SCPC Microalgae Combination
Our fourth suggested combination is the four microalgae, including S (Scenedesmus quadricauda), C (Chlorella pyrenoidosa), P (Parachlorella kessleri), and C (Chlamydomonas mexicana), that completely remove these 9 contaminants, according to Table 5. Scenedesmus quadricauda is able to remove many pollutants, such as heavy metals, antibiotics or pharmaceuticals, PAHs, pesticides, herbicides, and manure. The successful application of this microalgae combination is a crucial method in Pharmaceuticals Wastewater Treatment. Heavy metals, antibiotics or pharmaceuticals, and surfactants are removed by Chlorella pyrenoidosa, while nutrients can be removed by Parachlorella kessleri. Chlamydomonas mexicana bioremediated pesticides and insecticides. The composition of microalgae, including Scenedesmus quadricauda, Chlorella pyrenoidosa, Parachlorella kessleri, and Chlamydomonas mexicana, in the removal of 9 pollutants from ECs is presented in Table 5. Other advanced methods, such as Ozone Water Treatment Systems, are also effective as an alternative to Microalgae Combination in this area.
Table 5. ECs removal by microalgal strains
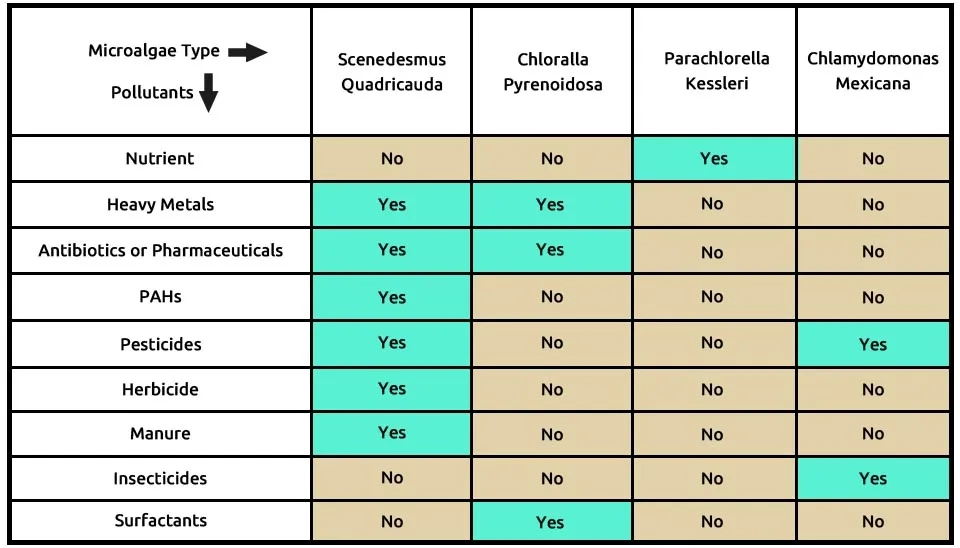
Click this link to see the full specification table
Table 5. ECs removal by microalgal strains
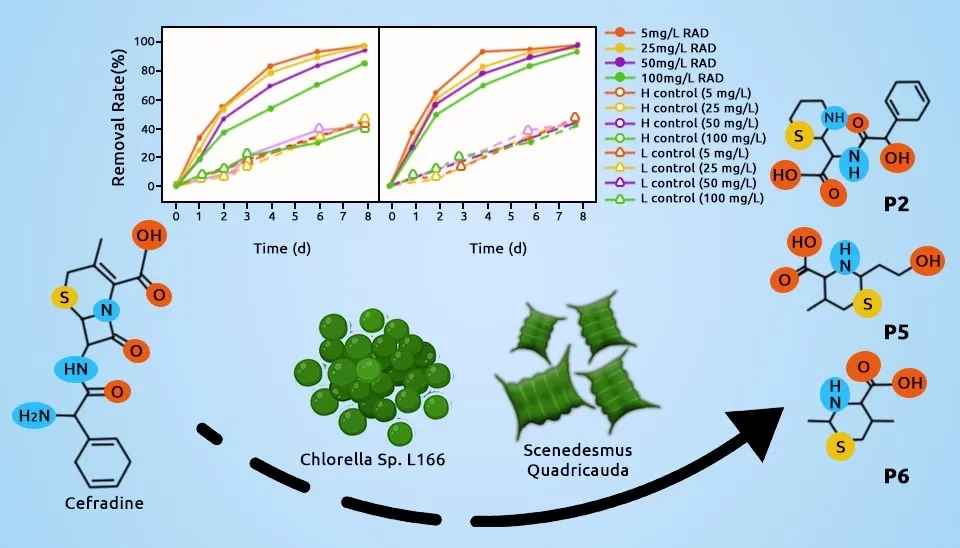
Anastopoulos and Z. Kyzas. (2015) stated that Scenedesmus quadricauda is another microalga effectively used for the sequestration of Cr³⁺ and Cr⁶⁺ ions, with removal efficiencies ranging from 51% to 96%. This process was carried out over a pH range of 1–9 and with a contact time of 120 minutes. Tan et al. (2018) investigated the growth rate and nutrient uptake of Scenedesmus quadricauda microalgae in swine manure wastewater. They specifically focused on the removal of total phosphorus, ammonia nitrogen, nitrate nitrogen, and nitrite nitrogen during the cultivation process. The results demonstrated that Scenedesmus quadricauda exhibited good growth in swine manure wastewater. S. quadricauda reduces 83.99% of Chemical Oxygen Demand (COD) and 80.39% of Biochemical Oxygen Demand (BOD5) total phosphorus (84.78%), ammonia nitrogen (91.79%), nitrate nitrogen (89.79%), and nitrite nitrogen (87.14%). Scenedesmus quadricauda grows at room temperature of 30 ± 1°C with a continuous light supply at about 1520 Lux. Wang et al. (2022) investigated cefradine antibiotic removal using Scenedesmus quadricauda , achieving a removal efficiency of 98.50% in 8 days. The microalga was cultivated under a light intensity of 5000 ± 100 Lux and at a temperature of 25 ± 1°C, demonstrating its strong potential for antibiotic removal in environmental applications.
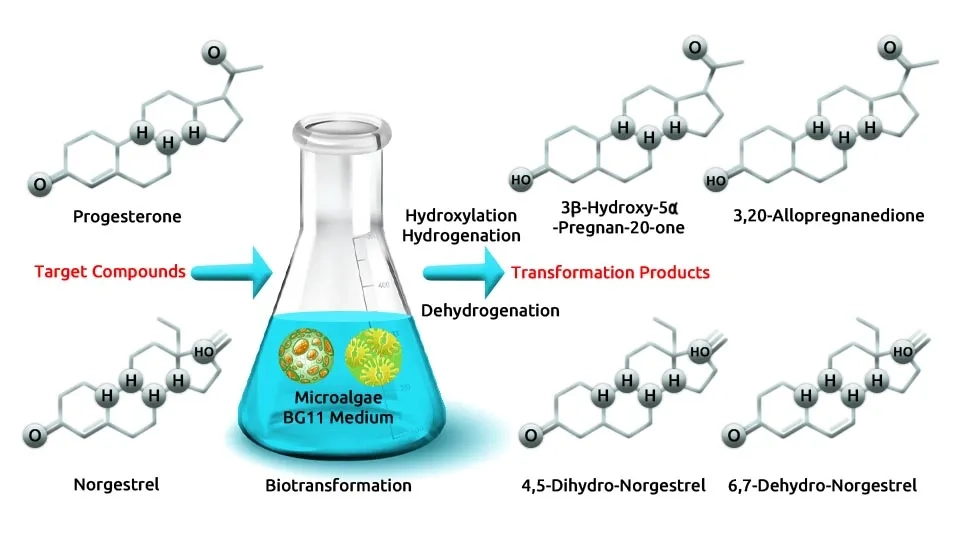
Guo and Chen. (2015) evaluated the effectiveness of the freshwater green alga Chlorella pyrenoidosa in removing cephalosporin antibiotics (including cefradine, cefalexin, ceftazidime, and cefixim). The study achieved a removal efficiency of 89–100% within 48 hours, showcasing the potential of C. pyrenoidosa for removing various cephalosporin antibiotics from aquatic environments. Chlorella pyrenoidosa was pre-cultured in BG-11 medium at a temperature of 25.0 ± 1.0°C under an illumination of 4000 lux, with a light-dark cycle of 12:12 hours. Wang et al. (2022) investigated the effects of varying dosages of sodium Lignosulfonate (LS), Sodium Dodecyl Sulfate (SDS), and Tween-80 surfactants on removal efficiency by Chlorella pyrenoidosa, achieving 100% removal. The microalgae Chlorella pyrenoidosa were cultured in a BG-11 medium in a 300-mL working volume under continuous light intensity of 200 µmol m-2 s-1, 25 ± 2°C, and aeration with air containing 2% CO2. Peng et al. (2014) investigated the biotransformation of progesterone and norgestrel pharmaceuticals removed (60–95%) in aqueous solutions by Chlorella pyrenoidosa and elucidated their transformation mechanisms at 5 days. Chlorella pyrenoidosa was cultivated in BG-11 medium under controlled conditions in a shaking incubator at a speed of 150 rpm and a temperature of 25 °C. The microalgae were illuminated with 3000 lux fluorescent light following a 12 h:12 h light/dark cycle, promoting optimal growth for the study.
Koutra et al. (2019) demonstrated a significant decrease in both organic and inorganic components in municipal wastewater, achieving removal efficiencies of 90–100% using Parachlorella kessleri over an 18-day period. P. kessleri was cultivated in BG-11 medium at room temperature, maintained at 25 ± 2°C, with continuous fluorescent light exposure of approximately 25–370 μmol photons m−2 s−1 and a stable pH of 8 ± 0.5. Kumar et al. (2015) investigated acephate and imidacloprid insecticides at concentrations of 1, 5, 10, 15, 20, and 25 mg L−1 achieving removal efficiencies of 21–25% by Chlamydomonas Mexicana at a 12-day period. Chlamydomonas Mexicana was cultivated in a shaker incubator at 27°C and 150 rpm, under 14:10 h light/dark cycle using white fluorescent light at an intensity of 45–50 μmol photon m−2 s−1. Liu et al. (2016) investigated lipid production in two freshwater microalgae species, Scenedesmus quadricauda and Chlorella pyrenoidosa. A concentration of 60 mg L-1 indole-3-propionic acid (IPA) significantly enhanced growth, showing a rate three times higher than the control. Additionally, fat production in samples treated with this auxin was generally greater compared to untreated samples.
Lv et al. (2019) investigated the growth and nitrogen removal abilities of five commonly used green algae species—Chlorella vulgaris, Chlorococcum sp. GD, Parachlorella kessleri TY, Scenedesmus obliquus, and Scenedesmus quadricauda—when cultivated in synthetic wastewater. After 7 days, all microalgae demonstrated high nitrogen removal efficiency, achieving 85.30–97.03% for ammonium and 100% for nitrate. Notably, Chlorococcum sp. and P. kessleri removed 85–90.24% of ammonium and 100% of nitrate within just 2–3 days, performing faster than the other three microalgae. Krienitz et al. (2003) studied the characteristics and morphology of Chlorella sp. and Parachlorella sp. Finding that traditional classification based solely on shape may not always be accurate. For example, traditional morphological criteria used for classification—such as spines, mucilaginous envelopes, or the formation of colonies or connubia—can vary significantly due to phenotypic plasticity. These traits help microalgae adapt to environmental factors by optimizing buoyancy in the water column and resisting physical pressure. Thus, identifying the optimal cultivation conditions for each microalgae strain in this suggested combination is essential.
5. SSCA Microalgae Combination
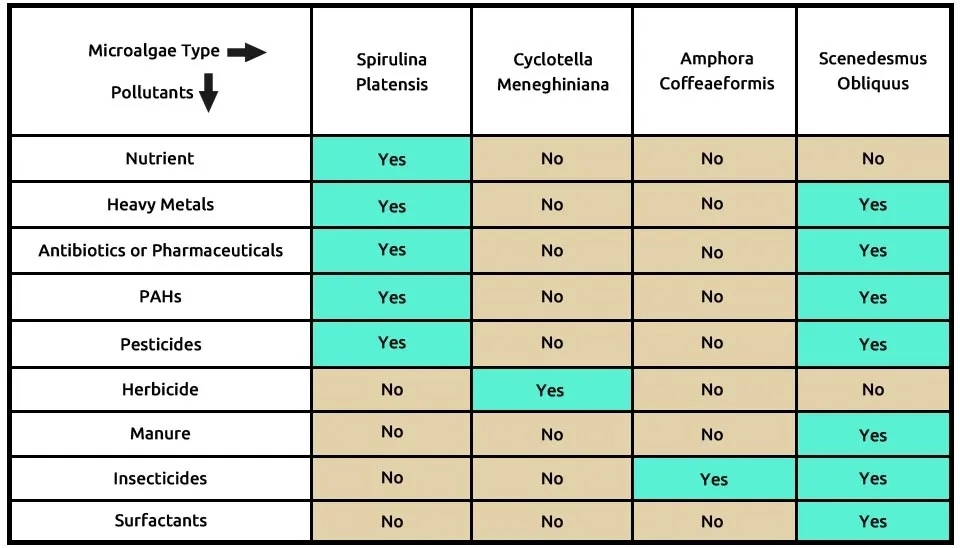
The fifth recommended combination utilized two robust microalgae, S (Spirulina platensis) and S (Scenedesmus obliquus), along with C (Cyclotella meneghiniana) and A. This combination achieved complete removal of the nine contaminants listed in Table 6. Spirulina platensis and Scenedesmus obliquus are effective at removing various pollutants, such as nutrients from municipal wastewater, heavy metals, antibiotics or pharmaceuticals, PAHs, pesticides, insecticides, surfactants, and manure. Cyclotella meneghiniana effectively removed herbicides, while Amphora coffeaeformis assists to removing insecticides. The composition of these microalgae—pirulina platensis, Scenedesmus obliquus Cyclotella meneghiniana, and Amphora coffeaeformis—in removing nine emerging contaminants (ECs) is shown in Table 6.
Table 6. ECs removal by microalgae strains
Click this link to see the full specification table
Table 6. ECs removal by microalgae strains
Zhai et al. (2015) demonstrated nutrients and wastewater treatment by cultivating microalgae in wastewater as a growth medium. Efficient harvesting of this microalgae biomass often involves techniques like Dissolved Air Flotation (DAF).
They grew Spirulina platensis in Synthetic Municipal Wastewater (SWW) under conditions of pH 8.8–8.9, the light intensity of 3300–3400 lx in with a 12-hour daily illumination period, a temperature of 25 ± 1°C and air-bubbling at 0.5 vvm. Under these conditions, Spirulina platensis 92.58% of total nitrogen (TN) and 94.13% of total phosphorus (TP). Abdel-Razek et al. (2019) achieved removal of 88–99% of organophosphate pesticides and heavy metals from water using a microalgae consortium of Chlorella vulgaris, Scenedesmus quadricuda, and Spirulina platensis over 28 days. The microalgae were incubated at 23 ± 1°C under a 24-hour light provided by a 25-watt bulb in each aquarium. Al-Homaidan et al. (2015) used Spirulina platensis to remove ions (Cd2+) achieving an 87.69% removal rate. The study varied conditions, including pH (3–9), biomass concentration (0.25–2 g), temperature (18–46 °C), metal concentration (40–200 mg/L), and contact time (30–120 minutes). Hassan et al. (2021) investigated the removal of lambda-cyhalothrin insecticide (20–100%) using Amphora coffeaeformis and Scenedesmus obliquus over 14 days. The microalgae were cultivated in an F2 medium containing 7 ppm nitrogen supplied as urea.
El-Bestawy et al. (1996) analyzed the elemental composition of Spirulina platensis and Cyclotella meneghiniana using electron-probe X-ray microanalysis. Despite high metal pollution in the lake, the microalgae absorbed a substantial amount of these metals. However, C. meneghiniana showed limited uptake of iron and copper.
A. Alwaleed et al. (2021) examined the effects of Spirulina platensis or Amphora coffeaeformis on growth performance, intestinal microbial population, physiological responses, and blood biochemical constituents in broiler chickens for two microalgae. Adding 10 g/kg of microalgae to the chickens' diet enhanced growth, improved biochemical parameters, and supported a healthier intestinal microbiome. A. El-Chaghaby et al. (2019) evaluated the antioxidant removal capacity, phytochemical constituents, and proximate composition of three microalgae, Scenedesmus obliquus, Chlorella vulgaris, and Spirulina platensis. The highest total antioxidant activity was observed in Spirulina platensis (3720.67 mg AAE/100 g), followed by Chlorella vulgaris (2794.80 mg AAE/100 g). The research on Spirulina platensis, Amphora coffeaeformis, Scenedesmus obliquus, and Cyclotella meneghiniana indicated that these microalgae strains can be cultivated effectively. It is suggested that optimal conditions for the cultivation of all examined microalgae strains be established to maximize their growth and performance.
6. CSANSNT Microalgae Combination
The sixth suggested combination includes seven microalgae species: C (Chlorella minutissima), S (Selenastrum capricornutum), A (Auxenochlorella protothecoides), N (Nannochloris oculata), S (Surirella brebissonii), N (Nitzschia palea), T (Tetraselmis suecica) which completely remove these nine contaminants, according to Table 7. Chlorella minutissima is effective in removing three pollutants, such as heavy metals, PAHs, and manure. Selenastrum capricornutum effectively removes Antibiotics or pharmaceuticals and PAHs, while other microalgae in the combination can each contribute to the removal of one of the nine targeted contaminants. The composition of microalgae—Chlorella minutissima, Selenastrum capricornutum, Auxenochlorella protothecoides, Nannochloris oculata, Surirella brebissonii, Nitzschia palea, and Tetraselmis suecica—in the removal of nine pollutants of ECs is presented in Table 7.
Table 7. ECs removal by microalgae strains
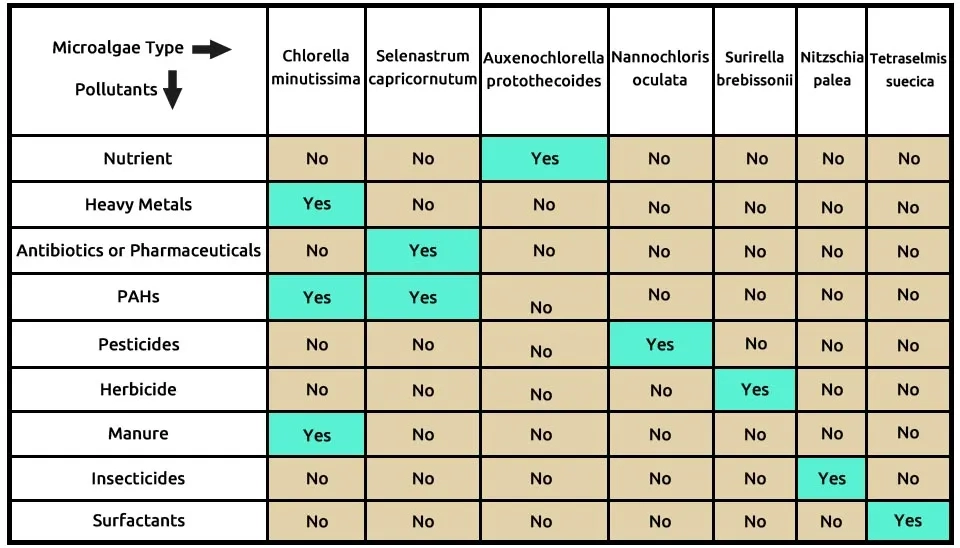
Table 7. ECs removal by microalgae strains
Click this link to see the full specification table
Khan et al. (2019) explored the biorefinery potential of Chlorella minutissima, Scenedesmus spp., and Nostoc muscorum, both individually and in consortium form. achieved notable removal efficiencies for various pollutants using the microalgae consortium, with reductions of 92% for NH₄⁺-N, 87% for NO₃⁻-N, 85% for PO₄³⁻-P, 96% for Total Dissolved Solids (TDS), 90% for Biochemical Oxygen Demand (BOD₅), and 81% for Chemical Oxygen Demand (COD) after 25 days. Microalgae were cultivated in Bold's Basal medium with solar radiation levels reaching 30.78 and 20.64 MJ m-2d-1 during the growth period.
Yang et al. (2015) developed an eco-friendly approach combining algae biofuel production with hazardous waste treatment. Chlorella minutissima demonstrated an ability to remove cadmium, copper, manganese, zinc ions, and heavy metals, achieving a removal efficiency of 62-44% under heterotrophic culture conditions over a 7-day period. C. minutissima was cultured in a 500 mL flask containing 200 mL artificial wastewater, illuminated by cool white fluorescent tubes at 50 μmol photon m−2 s−1, with a 12-hour light and 12-hour dark cycle. The pH range for cultivation was between 2 and 10, and temperatures tested included 10°C, 28°C, and 37°C, using 4 g L−1 of lyophilized biomass. Tam et al. (2010) removed Polycyclic Aromatic Hydrocarbons (PAHs) such as phenanthrene, fluoranthene, and pyrene, achieving removal rates of 73–92% over a 168-hour treatment period. S. capricornutum was cultured in a modified SE medium under axenic conditions and was harvested after 10–12 days.
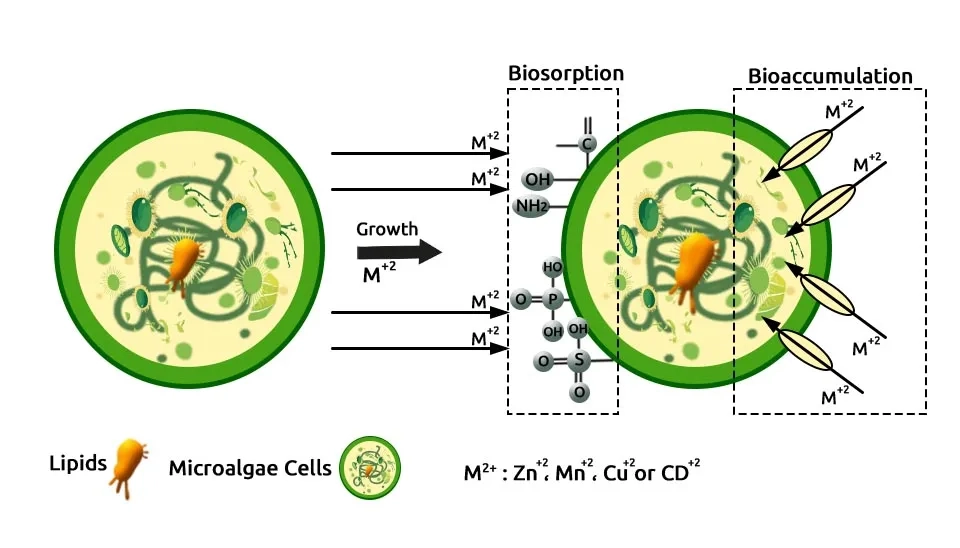
Hom-Diaz et al. (2015) explored the potential biodegradation of the hormones β-Estradiol (E2) and 17α-Ethinylestradiol (EE2) using two microalgae species, Selenastrum capricornutum and Chlamydomonas reinhardtii, in Anaerobic Digester Centrate (ADC). E2 and EE2 were effectively removed and achieving removal efficiencies between 88% and 100% for E2 and between 60% and 95% for EE2 after 7 days of treatment. S. capricornutum was cultivated in 100 mL Erlenmeyer flasks under continuous fluorescent lamp at an irradiance level of 172 ± 18 μmol photon m−2 s−1. The temperature was controlled at 25 ± 1°C with a shaking speed of 130 rpm. Zhou et al. (2012) investigated cost-effective cultivation methods by utilizing wastewater as a nutrient source for growing Auxenochlorella protothecoides over a 6-day batch cultivation period, optimizing nutrient removal efficiency and reducing production costs. The maximal removal efficiencies for TN, TP, COD, and total organic carbon (TOC) achieved by Auxenochlorella protothecoides were over 59%, 81%, 88%, and 96%, respectively.

Pérez-Legaspi et al. (2015) investigated the ability of microalgae Nannochloris oculatato to remove lindane , achieving a removal efficiency of 68–73% from the media within 7 days. Nannochloris oculatato was cultivated in Bold’s Basal Medium prepared with distilled water in 1 L flasks under continuous cold whitelight at an intensity of 59.91 μmol photon m−2 s−1 maintained at 18 ± 2°C. Constant aeration was provided by an aquarium air pump.
Wang et al. (2020) analyzed the response of Nitzschia palea to the insecticides acephate and trichlorfon, focusing on its physical, biochemical, and structural changes, along with its insecticide removal efficiency. Nitzschia palea removed 24.44–32.75% of insecticides after 7 days of cultivation. It was cultured at 20 ± 1°C with a light intensity of 4000 lx, following a 12:12 h light/dark period.
Sánchez-García et al. (2013) investigated the effect of nitrate on cell growth and lipid production, as well as the lipid composition of three microalgae strains: Monoraphidium contortum, Tetraselmis suecica, and Chlorella minutissima. The results showed that increasing nitrate concentration led to a reduction in lipid content and productivity in Monoraphidium contortum. However, an opposite effect was observed in Tetraselmis suecica and Chlorella minutissima cultures, where increased nitrate levels enhanced lipid production and content. C. minutissima and M. contortum lipids contain high levels of oleic acid, with values ranging from 26 to 45.7% and 36.4 to 40.1%, respectively. Ates et al. (2019) studied the impact of alpha-iron oxide (α-Fe2O3, 20–40 nm) and gamma iron oxide (γ-Fe2O3, 20–40 nm) Nanoparticles (NPs) on Selenastrum capricornutum and Nannochloropsis oculata. γ-Fe2O3 NPs inhibited the growth of N. oculata by 54% at a concentration of 0.2 mg/L. At a higher concentrations of 10 mg/L the nanoparticles caused high mortality rates, reaching 82% in Nannochloris oculata and 72% in Selenastrum capricornutum. Additionally, α-Fe2O3 NPs were found to be less toxic compared to γ-Fe₂O₃. At a concentration of 10 mg/L, α-Fe₂O₃ NPs induced a 97% mortality rate in Nannochloris oculata and strongly inhibited the growth of Selenastrum capricornutum by 73% at a concentration of 0.2 mg/L. Indeed, each microalgal species has distinct optimal growth conditions, such as nutrient requirements, light intensity, temperature, and pH. For a successful combination of multiple species in a bioremediation or treatment process, compatibility in these factors is crucial to avoid competition or inhibition. Conducting experimental trials would allow for a controlled assessment of their compatibility by testing various conditions to identify an environment where all species can co-exist and perform efficiently. This approach could help in developing optimized consortia tailored to target specific pollutants while maximizing pollutant removal efficiency.
Conclusion
Microalgae have demonstrated the ability to filter, concentrate, remove, or biotransform a range of emerging contaminants. This article introduces a microalgal consortium for the simultaneous removal of multiple ECs. The author suggests that this microalgal composition for contaminant removal should be investigated for the adaptation of cultivation conditions and selecting suitable microalgae species. Microalgae bioremediation can remove various types of wastewater, reducing carbon dioxide emissions and provide valuable applications of the produced biomass. In conclusion, using microalgae in biomonitoring and restoration of water systems contributes to environmental cleanup. It can be noted that most of the studies were conducted in batch reactors under controlled laboratory conditions. The impact of multiple ECs in wastewater in real-world applications is not well understood. According to the research, the proposed compositions of microalgae have removed all pollutants from that family examined in the research, but more detailed tests are needed to ensure the removal of all pollutants. In such situations, a pretreatment unit utilizing Membrane Filtration Technology may be necessary before a microalgae-based system may be needed to selectively remove pollutants prior to their treatment by the microalgae. Techniques like Microfiltration Technology and Ultrafiltration Technology are often used for this purpose. For complete water purification, advanced methods such as Reverse Osmosis might be applied post-treatment. The flow of operational conditions such as pH, temperature, dissolved oxygen, HRT, microalgae strain interactions, light limitation/inhibition, and mixing conditions in the real-world reactor system may significantly deter from laboratory conditions. Key challenges like understanding Membrane Fouling Types must be addressed. Therefore, pilot-scale studies are needed to address the challenges associated with the removal of ECs in this microalgae composition under dynamic and real-world environmental conditions.